Blockbuster Drugs In The Pharmaceutical Industry
        Analysis Of Top Five Blockbuster Drugs In The Pharmaceutical Industry
Abstract
Pharmaceutical industry is growing faster than many other industries in existence. Innovator drugs manufactured by the above industry enjoy a patent protection of around twenty years.
In my report I shall explain the difference between innovator and generic pharmaceutical industries and thereby compare and analyze the five highest
grossing drugs of all time. Further, I would compare their sale during their patent life and after the expiry of their patent life.
                  Introduction
In this technology driven world, the awareness of various brands has become common knowledge. Majority of the people identify brands like Apple, Samsung, Honda, Toyota, Tata, Vodafone and Reliance and some even possess decent knowledge about them. The industry with most unpopular brands is the pharmaceutical industry.
This doesn’t change the fact that pharmaceutical industry is making huge profits. With the spread of epidemics and diseases the pharmaceutical industry is blooming. Pharmaceutical drugs have a quite long patent life (around 20 years) than
products of other industries. During the patent life of a certain drug, the respective company enjoys monopoly. Awareness regarding pharmaceutical industry, their investment patterns and their profit generation is of utmost importance.
             Objective Of Study
- Â Â To determine the difference between Generic and Innovator drugs.
- Â Â Differentiate between drug, brand and company.
- Â Â To understand patent protect laws.
- Â Â To analyze the profit made by five highest grossing drugs in the world.
- Â Â To observe the sale patterns during patent life and post patent life.
- Â Â To understand the role of FDA in regulating the pharmaceutical industry.
       Pharmaceutical Industry
All the companies manufacturing medicines, injectable, inhalers, vaccines and core healthcare products come under the pharmaceutical industry.
Pharmaceutical Companies are broadly classified into two types.
- Â Innovator Companies
- Â Generic Companies
Innovator companies are those companies which do the tough job. They identify the disease, study the causing agent, develop a molecule, test the molecule, develop the drug, test it on animals, test it on humans, study the adverse effects, get the drug approved from FDA (Food and Drug Administration), manufacture it and finally sell it in the market.
The above process of developing a totally new drug requires an investment of 10 to 20 years of time and $1 billion to $10 billion of capital. Apart from the above labor, FDA take more 2 to 5 years to approve the drug. In return, the company gets a patent protection on the above product for around 20 years, if applied.
The company enjoys monopoly in the market as long as the patent of the product lasts. Of all the molecules developed, only 10% make to the market. The investment required in an innovator company is humongous.
Companies like Pfizer, Novartis, Amgen, AstraZeneca and Eli Lilly are Innovators.
Generic companies, on the other hand, play it safe. They don’t develop any molecule, instead they develop the innovator’s drug at much lower cost. When the innovator drug’s patent expires, the generic companies flood the market with their drugs at a much lower price. Since generic companies don’t have a patent protection, they have to face competition from the other generic companies as well as the innovator itself.
Almost all Indian pharmaceutical companies are generic in nature.
Companies like Sandoz, Ranbaxy, Cipla, and Teva are Generic.
                     Products
There are 3 terms used while talking about pharmaceutical products are drug also known as molecule or generic name, the brand name and the company. I’ll differentiate between the 3 using the example of a common medicine called Crocin.
- Drug/Molecule/Salt/Generic Name – It denotes the composition of the product, here medicine. Many medicines can have the same generic name but not the same brand name.
Example: Acetaminophen
- Brand/ Brand Name – It is the unique name given by the company to its product. No two brand name can be the same.
Example: Crocin
- Company – It is the organization that manufactures the drug and sells it in the market.
Example: GlaxoSmithKline
Patent and Intellectual Property
A Patent is a commitment by the Government and WTO (World Trade Organization), ensuring the owner monopoly of a certain entity for a particular period of time. Any person or organization must take permission from the patent owner to reproduce a part or whole of his patented entity, failing which the patent owner holds the power to sue the certain individual or the organization in the court of law.
The number of patents issued in the pharmaceutical industry far exceeds the number of patents issued in any other industry. There are 2 types of patents.
- Design Patents
- Utility Patents
In the pharmaceutical industry even the process can be patented calling it a process a patent. All countries which have signed the charter with WTO are obliged to respect the charter. There are 3 ways to sell the patented entity within its patent life.
- Receive permission from the current patent owner.
- Purchase the patent rights from the owner.
- Bypass the patent, develop a different process to develop an entity from the one patented.
Top 5 Highest Grossing Drugs
- Â Lipitor by Pfizer
- Â Plavix by Sanofi and Bristol – Myers
- Â Humira by AbbVie
- Â Seretide by GlaxoSmithKline
- Â Enbrel by Amgen
- Â Â Â Brand: Lipitor
            Drug: Atorvastatin
            Company: Pfizer
Lipitor was launched by Pfizer in 1997 and it has been the highest grossing drug of all time. It made sales of around $120 billion until its patent expired in 2011. Now Lipitor sales have declined due to facing competition by generic industries.
The above graph shows that Lipitor sales were at its peak between 2006 and 2009. After the expiry of the patent in 2011, its sales have considerably declined.
Lipitor was used to reduce cholesterol in the blood. Due to the birth of junk food in early 2000, the cholesterol level in majority of the population increased, owing to high sales of Lipitor during that period.
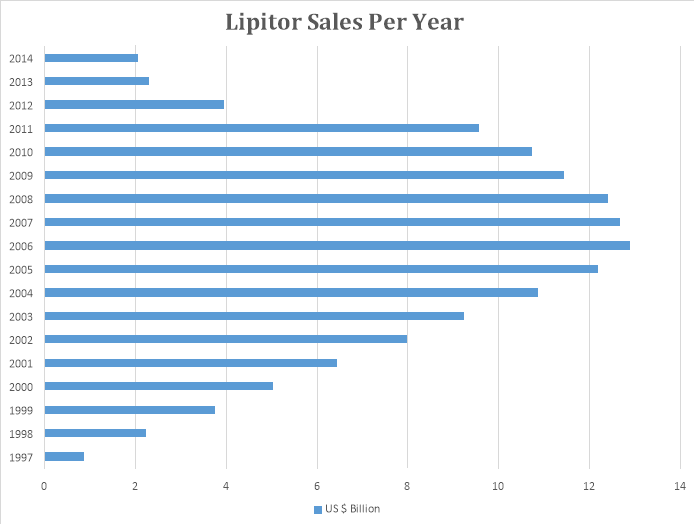
Pfizer had a golden time during the patent tenure of Lipitor. Even today, Pfizer is the largest innovator company in the world.
- Brand:Â Plavix
Drug:Â Clopidogrel
Company:Â Bristol – Myers & Sanofi
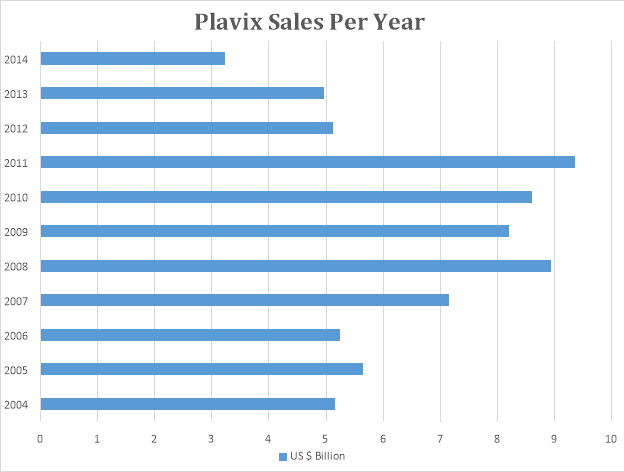
The above graph depicts the annual sales of Plavix during its patent life. Plavix was launched in 1998 as a joint effort by Sanofi Aventis and Bristol Myers. Plavix patent will expire by 2016. It is the second bestselling drug in the world after Lipitor.
Plavix is used to prevent and avoid heart attacks in patients. Plavix has an all-time sale of $ 87 Billion.
- Brand: Humira
Drug: Adalimumab
Company: AbbVie
Humira was launched by AbbVie in 2003. It is one of most best-selling drug of the 21st century. It was developed for the treatment of Rheumatoid Arthritis. Its patent is still intact. The graph below denotes the sale of Humira.
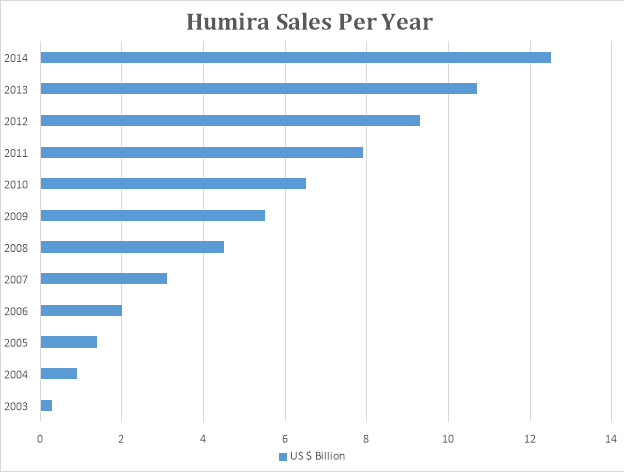
From 2003, when Humira was launched, its sales have increased exponentially and it still has patent protection. If this trend continues, it might rival Lipitor.
- Brand: Seretide
Drug: Salmeterol and Fluticasone
Company: GlaxoSmithKline
Seretide was first launched in Europe in 1999. Seretide was sold along with an inhaler. It was developed to prevent people from dying from an asthma attack. The use an inhaler to absorb medicine was a revolutionary concept in late 1990s.
Many generic alternatives of Seretide are available in the market today, due to the patent expiry of Seretide. Seretide was one of the highest grossing medicine of all time.
The graph below depicts sale patterns of Seretide through different years.
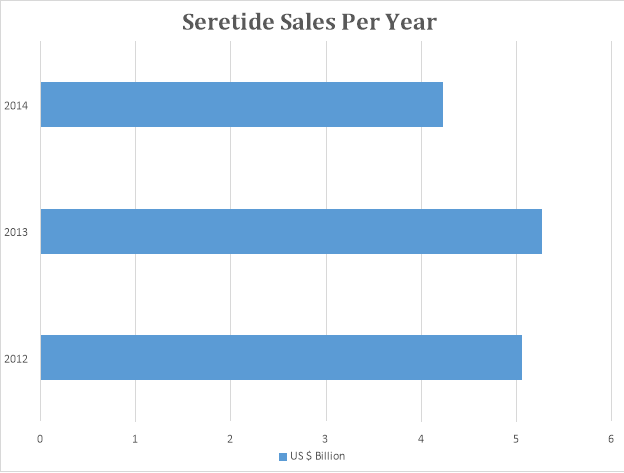
- Brand: Enbrel
Drug: Etanercept
Company: Amgen
The graph below depicts the sale pattern of Enbrel.
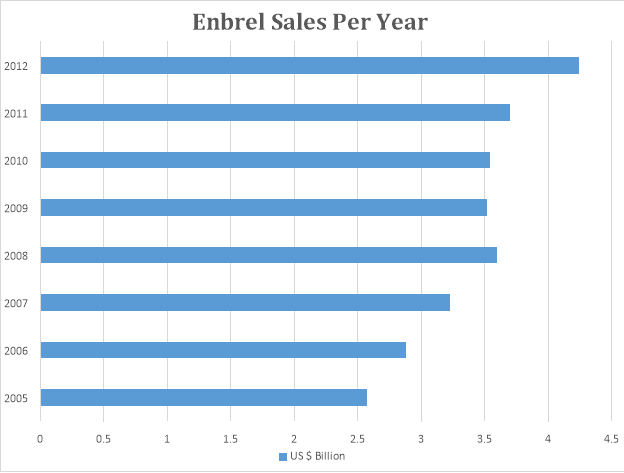
Enbrel was launched in 2005 by Amgen. It was a bio pharmaceutical, only one of its kind. It was developed as a cure for rheumatoid arthritis and became one of the most bestselling bio drug in the world. The Enbrel sale is on the rise.
Food and Drug Administration
Also known as FDA, it is a government body which is responsible for regulating the pharmaceutical industry. No company can sell a product as long as it is not approved by FDA.
FDA spends billions of dollars on testing and approving a drug. The entry barrier for pharmaceutical industries is quite high due to which only quality companies can survive.
FDA holds the right to make organization withdraw their product from the market. Even generic drugs need an FDA approval before they make to the market.
FDA discourages consumers to consume food supplements which are sold online, as selling a product online doesn’t require an FDA approval. FDA has the final word in the entire pharmaceutical industry.
                       Result
It is clear that the sales of a certain product rise and reach a peak point during the tenure of its patent life. The sales of the products sharply decline after the expiry of the patent. In other words, the sales of a certain product during its patent life form a rough parabola.
The revenues earned by the brands in their patent life decrease in the following order.
Lipitor -Â Â $125 Billion
Plavix -Â Â $ 87 Billion
Humira -Â $ 11.8 Billion and Ongoing, Patent Intact
Seretide - $ 18 Billion
Enbrel - $ 8.7 Billion and Ongoing, Patent Intact
                    Conclusion
Having compared the highest grossing products of the pharmaceutical industry, it is now evident that although the investment in pharmaceutical industry is enormous, the return on investment is even great. Patent and Intellectual property laws play a huge part in regulating the pharmaceutical; industry.
It is recommended that every country should sign the WTO charter and respect intellectual property laws as their own legal system. Many companies have started developing both innovator and generic drugs to survive the competition. With time as pathogens evolve, we’ll need stronger and better drugs to ensure the sustenance of the human race.