Effect of the Inorganic Filler Contents on Polymer
ANALYSIS OF ZIF 8/PAI AND CMS/PAI MEMBRANES FOR CO2/CH4 GAS
SEPARATION
Yohannan Subin Sabilon
Department of Chemical Engineering, National Institute of Technology Tiruchirappalli, India
Zeolitic Imidazolate Frameworks 8 (ZIF 8) nanocrystals and Carbon Molecular Sieves (CMS) particles were prepared by using standard procedures. UV visible spectroscopy and XRD tests were done for the confirmation of the particles prepared and scanning electron microscopy (SEM) and high resolution transmission electron microscopy (HRTEM) analysis were done to study the morphology of the particles prepared. ZIF 8/PAI and CMS/PAI MMMs were successfully synthesized by using ZIF 8 and CMS inorganic fillers and Polyamideimide (PAI) polymer using phase inversion technique. Various weight contents (1%, 2% and 3%) of the inorganic fillers were incorporated in the polymer matrix. Reinforcing of the polymer matrix with inorganic fillers was done in the form of nano and micro particles respectively. The effect of the inorganic filler contents on the mechanical properties of the polymer was investigated. Hydrophilic nature and porosity determination test, Fourier transform infrared spectroscopy (FTIR) and SEM were done to study the hydrophilicity and morphology of the composite system.
Keywords: Carbon dioxide, Methane, Mixed Matrix Membranes, Carbon Molecular Sieves,Zeolitic Imidazolate Frameworks
INTRODUCTION
Carbon dioxide (CO2) is one of the components of landfill gas, natural gas and biogas. It is also the main combustion product of fossil fuels and a leading contributor to global warming as it’s a greenhouse gas. In order to obtain fuel with enhanced energy content, to prevent corrosion problems in the gas transportation system and to reduce the climatic impact of CO2 gas it is quite essential to remove CO2 from those gas streams. This has driven the development of different technologies for CO2 gas separation. Among the different types of technologies being used membrane technology has experienced substantial growth, breakthroughs and advances during past decades [10]. Membrane technology offers high energy efficiency, simplicity in design and construction of membrane modules and environment compatibility. Although there are different types of membranes being used the combination of the superior performance of inorganic materials with the handling properties of the polymers is offered by Mixed Matrix Membranes (MMMs). Therefore in our study we will be using MMMs for CO2/CH4 gas separation. In the MMMs the inorganic fillers are added to the polymer matrix. Over the years different inorganic fillers have been used for preparing MMMs for CO2/CH4 gas separation out of which Zeolitic Imidazolate Framework 8 (ZIF 8) is known to show maximum selectivity while Carbon Molecular Sieves (CMS) is known to show maximum permeability [19]. In this study the preparation and characterization of these inorganic fillers is shown. These inorganic fillers were successfully incorporated in the Polyamideimide (PAI) polymer matrix and MMMs were prepared. The characterization and analysis of the ZIF 8/PAI and CMS/PAI MMMs have been done with different loading of inorganic fillers in order to choose the best possible membrane combination for CO2/CH4 gas separation.
EXPERIMENTAL SECTION
Materials
Zinc hydrate crystals and N-methyl 2-pyrrolidone (NMP) required in the preparation of ZIF 8 nanocrystals were purchased from Merck Life Science Private Limited, Mumbai, India. Methanol used for washing during centrifugation was also bought from Titan Biotech Limited, Rajasthan, India. 2-methylimidazole and n-butylamine also required for the preparation of ZIF 8 nanocrystals were bought from Otto Group Hamburg, Germany, Polyamidieimide polymer was also purchased from UTM, Malaysia. Acetone was purchased from Merck Specialities Private Limited, Mumbai, India. All reagents were used without any further purification.
Synthesis of ZIF 8 nanocrystals
ZIF 8 nanoparticles were synthesized based on the procedure reported by Cravillon et al[3]. The ZIF-8 nanocrystals so formed was sent for UV spectroscopy, XRD, HRTEM and SEM analysis.
Synthesis of CMS particles
CMS particles were synthesized based on the procedure reported by De. Q. Vu et al[8]
The CMS particles were then sent for XRD analysis.
Synthesis of ZIF 8/PAI membranes
Membranes of 3 different concentrations i.e., 1%, 2% and 3% of ZIF 8 nanocrystals were prepared by solution casting method. 17wt% of polyamideimide polymer solution was prepared by dissolving exactly 5.274g mixture of polyamideimide polymer i.e., Torlon and ZIF 8 nanocrystals in 25ml of NMP solvent in a beaker. A magnetic bead was cleaned and dried using acetone and was placed in the beaker. The 3 beakers containing the 3 different concentration solutions were kept on 3 different magnetic stirrer for complete dissolution. The exact amount of polymer and inorganic filler taken for respective concentrations is given in the table below:
Table 1 Composition of ZIF 8/PAI membranes
|
Concentration of ZIF 8/PAI |
Amount of PAI (g) |
Amount of ZIF 8 |
|
membranes (wt %) |
nanocrystals (g) |
|
|
1 |
5.116 |
0.158 |
|
2 |
5.169 |
0.105 |
|
3 |
5.221 |
0.053 |
Now 3 glass plates and casting rods were washed and kept for drying. After drying the glass plates and the casting rods were cleaned and dried by using acetone. After complete dissolution the polymer solution in the 3 beakers were casted on 3 different glass plates using casting rods of 750 µm thickness. The glass plates after casting were allowed to dry at room temperature overnight for all the NMP solvent to evaporate. After drying the polymer membrane so formed was peeled off the glass plate. The membrane samples were sent for SEM analysis.
Synthesis of CMS/PAI membranes
Membranes of 3 different concentrations i.e., 1%, 2% and 3% of CMS particles were prepared by solution casting method. 17wt% of polyamideimide polymer solution was prepared by dissolving exactly 5.274g mixture of polyamideimide polymer i.e., Torlon and CMS particles in 25ml of NMP solvent in a beaker. The exact amount of polymer and inorganic filler taken for respective concentrations is given in the table below:
Table 2 Composition of CMS/PAI membranes
|
Concentration of ZIF 8/PAI |
Amount of PAI (g) |
Amount of CMS particles |
|
membranes (wt %) |
(g) |
|
|
1 |
5.116 |
0.158 |
|
2 |
5.169 |
0.105 |
|
3 |
5.221 |
0.053 |

A magnetic bead was cleaned and dried using acetone and was placed in the beaker. The 3 beakers containing the 3 different concentration solutions were kept on 3 different magnetic stirrer for complete dissolution. Now 3 glass plates and casting rods were washed and kept for drying. After drying the glass plates and the casting rods were cleaned and dried by using acetone. After complete dissolution the polymer solution in the 3 beakers were casted on 3 different glass plates using casting rods of 750 µm thickness. The glass plates after casting were allowed to dry at room temperature overnight for all the NMP solvent to evaporate. After drying the polymer membrane so formed was peeled off the glass plate. The membrane samples were sent for SEM analysis.
TESTING AND CHARACTERIZATION
Confirmation tests for inorganic filers
UV visible spectroscopy analysis. The ultraviolet-visible spectroscopy (UV-Vis)utilizes light to determine the absorbance or transmission of a chemical species in either solid or aqueous state. The UV Visible Spectroscopy analysis was done for the confirmation of ZIF 8 nanocrystals.
XRD analysis. XRD can be done on a number of different kinds of samples. Smallvolume of sample was tapped on microscope slide glass. The intensity of the beam used was 40 kV and 40 mA. The XRD analysis was done for the confirmation of ZIF 8 nanocrystals and CMS particles.
Morphological studies of Inorganic fillers and MMMs
SEM with EDX analysis. The surface morphology of PAI polymer was observed usingthe JSM-6701F with high resolution field-emission scanning electron microscopy (FE-SEM) with the magnification of 5000Ã-. For EDX analysis the acceleration voltage was set to 20kV and the working distance was set to 14mm. The detector was moved down to 45mm. The sample was scanned by X-rays for a time of 200s. The elemental analysis of film in order to confirm the presence of carbon was done using an energy dispersive X-ray spectrometer (EDX) with magnification of 3000Ã- and acceleration voltage of 15 kV. After the scan was completed the spectrum was plotted using the data obtained from the scan. SEM with EDX was done for the confirmation of the CMS polymer film.
TEM analysis. The sample preparation was done by sputtering the same with gold.Then the chamber door was opened and the sample was placed in the sample holder. The chamber door was closed and the required input like voltage, acceleration and time for scan were given to the system connected to the TEM analyzer. The scan was started and the results were recorded. TEM analysis was done for the size determination of the ZIF 8 nanocrsytals.
FTIR analysis. Fourier transform infrared spectroscopy (FTIR) is a technique whichis used to obtain an infrared spectrum of absorption or emission of a solid, liquid or gas. An FTIR spectrometer simultaneously collects high spectral resolution data over a wide spectral range. Sulfonic acid group functionality of membrane was studied using attenuated -total-reflectance Fourier transform infrared (ATR-FTIR) spectroscopy (Thermo scientific Nicolet iS5 FTIR spectrometer). The spectra for all dried membranes were observed from the range from 4000 to 400 cm-1 wavelength.
Mechanical strength test
The material strength of the membranes prepared were studied by the performing Stress-Strain tests. The Universal Testing Machine was used to perform the tests. The samples of the membranes were cut into dimensions of height 30mm, width 10mm and thickness 0.45mm. The initial gauge length was set at 20mm. The samples were placed in a sample holder one at a time and the tests were performed. The data was recorded and the graphs were plotted for respective samples.
Hydrophilic nature and Porosity determination test
The hydrophilic or hydrophobic nature of the membranes were studied by immersing a 1cmx1cm membrane samples in different beakers each containing 20ml water. The beakers were kept on a rotary shaker for continuous mixing overnight. After 24 hours the membrane samples were taken out and the weight of the wet membranes were measured using a digital weighing balance. After that the membranes samples were dried in a vacuum oven at 60oC for 6 hours and then the weight of the dry membranes were measured similarly. The amount of water absorbed and the average porosity of the membranes were determined and the results were tabulated.
RESULTS AND DISCUSSION
Confirmation of ZIF 8 Nanocrystals
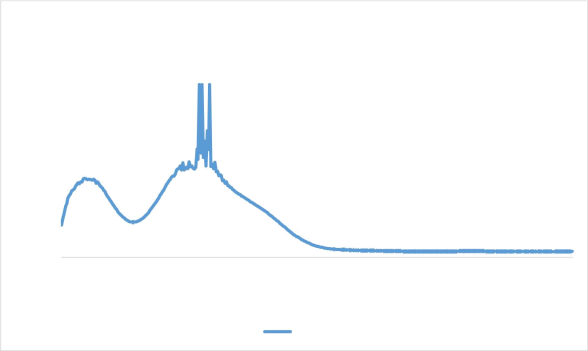
The UV Visible Spectroscopy analysis was done for the primary confirmation of ZIF 8 nanocrystals.
|
UV of ZIF 8 nanocrystals |
|||||||||||||||||||||||||||||||||
|
12 |
|||||||||||||||||||||||||||||||||
|
10 |
|||||||||||||||||||||||||||||||||
|
Absorbance |
8 |
||||||||||||||||||||||||||||||||
|
6 |
|||||||||||||||||||||||||||||||||
|
4 |
|||||||||||||||||||||||||||||||||
|
2 |
|||||||||||||||||||||||||||||||||
|
200 |
212 |
224 |
236 |
248 |
260 |
272 |
284 |
296 |
308 |
320 |
332 |
344 |
356 |
368 |
380 |
392 |
404 |
416 |
428 |
440 |
452 |
464 |
476 |
488 |
500 |
512 |
524 |
536 |
548 |
560 |
572 |
584 |
596 |
|
Wavelength |
|||||||||||||||||||||||||||||||||
|
Series1 |
|||||||||||||||||||||||||||||||||
Figure 1 UV visible spectroscopy result of ZIF 8 nanocrystals
The penetration depth was found to be directly proportional to the exciting wavelength i.e., 325nm because of decreased absorbance which is in accordance with the reference paper, Liu et aL, (2013)[1]. Therefore we can confirm that its ZIF 8 nanocrystals.
The XRD analysis was done for the secondary confirmation of ZIF 8 nanocrystals.
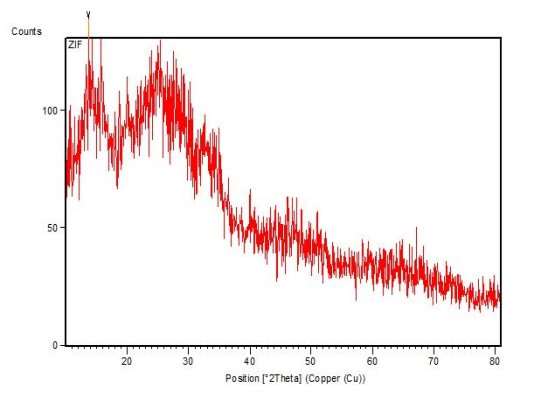
Figure 2 XRD result of ZIF 8 nanocrystals
When n-butylamine is added as the modulating ligand, nearly instantaneous formation of a solid is observed upon combining the component solutions, and pure-phase ZIF-8 nanocrystals are recovered after 24 h (see XRD pattern in Figure 2). An average size of 18 nm is estimated from the broadening of the Bragg reflections. The XRD results were also in accordance with the reference paper Cravillon et aL, (2011). Hence we can confirm that the particles synthesized were ZIF 8 nanocrystals.
Morphology of ZIF 8 Nanocrystals
ZIF materials constitute a new distinctive, rapidly developing subclass of crystalline porous coordination polymers (PCPs) or metal organic frameworks (MOFs). The tetrahedral framework structures of ZIFs are constructed from bivalent metal cations and bridging substituted imidazolate anions and frequently possess a zeolite topology. Numerous ZIFs combine the attractive features of MOFs (diversity of framework structures and pore systems, large surface areas, post-synthetically modifiable organic bridging ligands) with high thermal and chemical stability.
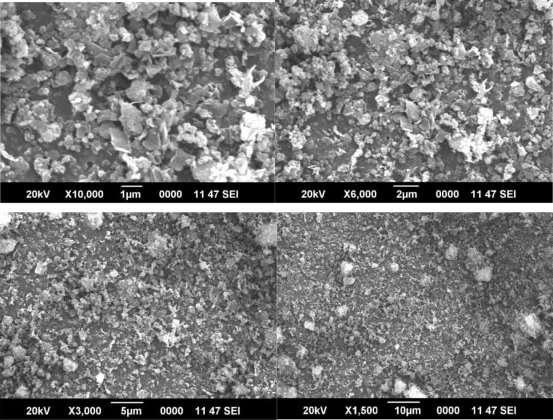
Figure 3 SEM image of ZIF 8 nanocrystals
It is this combination of properties which makes ZIFs very promising candidate materials for many technological applications. Properties and performance of porous materials
rely much on their supply as nano and microcrystals of well-defined size and shape, as is well-known for zeolites. SEM images (Figure 3) reveal that the well-defined nanocrystals have a rhombic dodecahedral shape crystal structure.
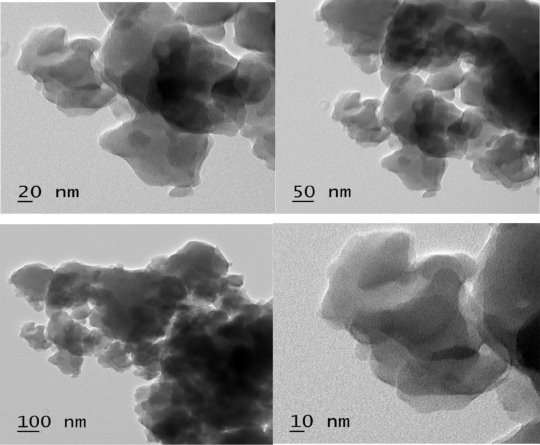
Figure 4 TEM image of ZIF 8 nanocrystals
TEM images (Figure 4) show roughly spherical particles being <100 nm in size, including some isolated particles and particles with sharp edges. It should be noted that the small ZIF nanocrystals are very sensitive to the high energy of the electron beam of a TEM.
Confirmation of CMS Particles
It is not possible to directly measure permeation properties of CMS particles as with CMS films, replicate mixed matrix films prepared with the two different sieves give very similar effective mixed matrix film permeation properties using powder-pyrolyzed sieves versus the film-pyrolyzed sieves. XRD was performed on the CMS films and powder, as shown in Fig. 5. The XRD diffractograms show very similar peaks and d-spacings, suggesting similar planar dimensions between the film-pyrolyzed CMS and the powder-pyrolyzed CMS, thereby confirming that the particles produced were CMS particles.
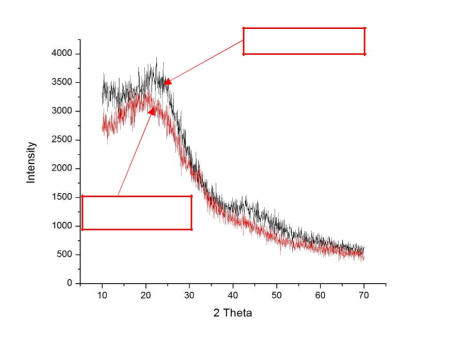
CMS particles
Polymer film
Figure 5 XRD results of CMS particles and CMS polymer film
Surface Morphology of CMS Polymer Film
The CMS membrane films have an intrinsic CO2/CH4 selectivity of 200 with a CO2 permeability of 44 Barrers at 35oC. For Ultem®-CMS mixed matrix membrane films, pure gas permeation tests show enhancements by as much as 40% in CO2/CH4 selectivity over the intrinsic CO2/CH4 selectivity of the pure Ultem® polymer matrix. Likewise, for Matrimid®- CMS mixed matrix films, enhancements by as much as 45% in CO2/CH4 selectivity were observed. Effective permeabilities of the fast-gas penetrants (O2 and CO2) through the mixed matrix membranes were also significantly enhanced over the intrinsic permeabilities of the Ultem® and Matrimid® polymer matrices. These encouraging selectivity and permeability enhancements confirm that mixed matrix membrane behaviour is achievable with CMS particles.
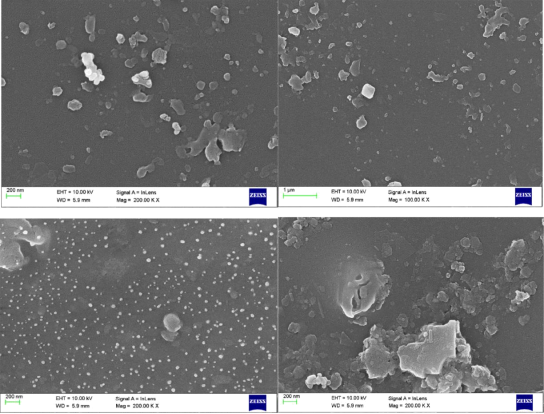
Figure 6 SEM image of CMS polymer film
Fig. 6 shows top surface SEM micrographs of a CMS polymer film. These micrographs show a smooth surface without any defects.
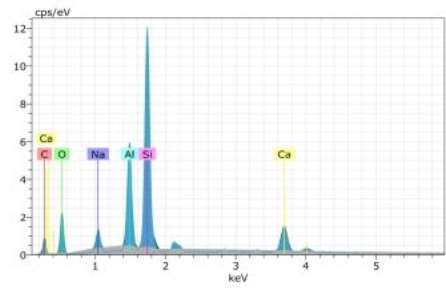
Figure 7 EDX result of CMS polymer film
The table below shows the EDX analysis of the CMS polymer film. The sharp Silicon peak is present due to the Silicon detector used during the EDX analysis.
Table 3 EDX result of CMS polymer film
|
Element |
Series |
unn. C |
norm. C |
Atom. C |
Error (3 |
|
[wt.%] |
[wt.%] |
[at.%] |
Sigma) |
||
|
[wt.%] |
|||||
|
Carbon |
K-series |
8.50 |
23.61 |
36.45 |
4.40 |
|
Oxygen |
K-series |
9.89 |
27.46 |
31.82 |
4.26 |
|
Sodium |
K-series |
1.16 |
3.22 |
2.60 |
0.31 |
|
Aluminium |
K-series |
4.56 |
12.67 |
8.70 |
0.74 |
|
Silicon |
K-series |
9.37 |
26.03 |
17.19 |
1.28 |
|
Calcium |
K-series |
2.52 |
7.01 |
3.24 |
0.31 |
|
Total: |
36.01 |
100.00 |
100.00 |
The Oxygen peak is due to the oxygen present in the atmosphere during EDX analysis. The Carbon peak denotes the confirmation of the CMS polymer film prepared. As expected it shows a maximum wt % of 23.61.
Cross Sectional Morphology of CMS/PAI Membranes
Scanning electron micrographs of the CMS fibers are shown in figures 8, 9 and 10
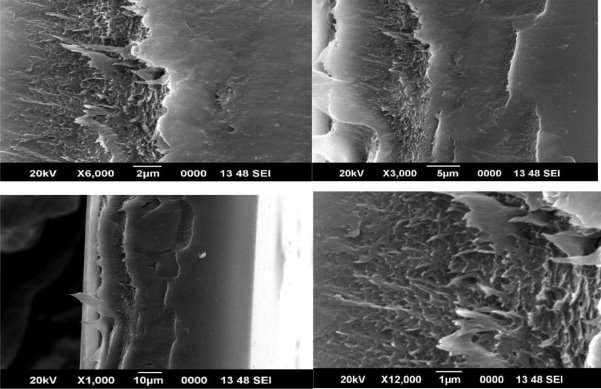
Figure 4.8 SEM image of 1% CMS/PAI membrane
Although asymmetry appeared to be present in the CMS fiber morphology, the thicknesses of the layers were markedly different from each other and from those of the precursor fibers (compare with those of the precursor fibers in Figure 6). The original polymeric precursor fibers consisted of a very thin dense skin (1000-2000 Å) on a porous core. This skin layer in polymeric fibers has been observed at very high resolution under SEM. In figure 8, high magnification of the wall in the cross section of the PAI CMS fiber reveals a gradual transition from the porous inner core to the denser outer micropore structure. In contrast, high magnification of the PAI CMS fiber shows a uniform dense micropore structure in figure 9.
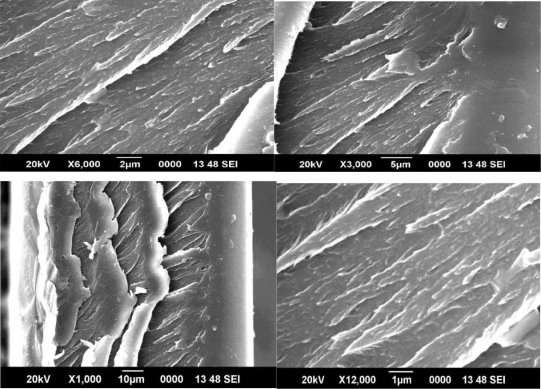
Figure 9 SEM image of 2% CMS/PAI membrane
Figures 8, 9 and 10 show SEM micrographs of a mixed matrix film after these modifications. These micrographs demonstrate smaller CMS particles (mostly <1µm) and a better distribution of these particles, as well as very good polymer-sieve contact.
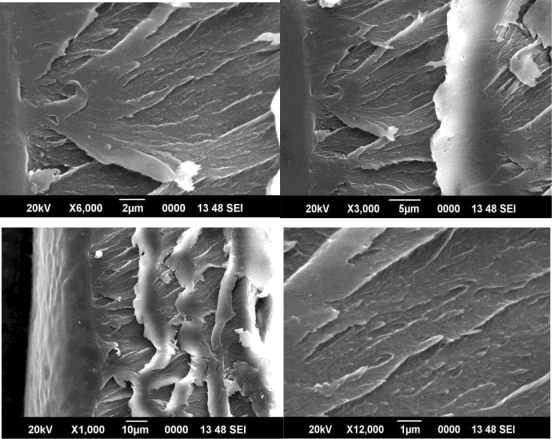
Figure 10 SEM image of 3% CMS/PAI membrane
Cross Sectional Morphology of ZIF 8/PAI Membranes
Figures 11, 12 and 13 shows SEM images of ZIF-8/PAI mixed matrix dense films, which indicates good contact of bare ZIF-8 to the PAI matrix without “sieve-in-a-cage” morphology at each loading. It is noteworthy that the good contact was achieved without any surface treatment of the sieve. This should be due to the hydrophobic nature of ZIF-8, proved by TGA measurements in reference paper Zhang et. al. (2012). Interestingly, in the SEM images of ZIF-8/PAI mixed matrix dense films, as shown in figures 11, 12 and 13, we observe a morphology that has not been previously reported in mixed matrix membranes prepared with other molecular sieves. Other than well-dispersed 10 nm ZIF-8 particles, there also exist many non-ideal large clusters of ZIF-8 with size ranging from 50 nm to several microns, which is more than an order of magnitude larger than single ZIF-8 particles. Also, volume fraction of large ZIF-8 clusters in the matrix increases with increasing ZIF-8 loading.
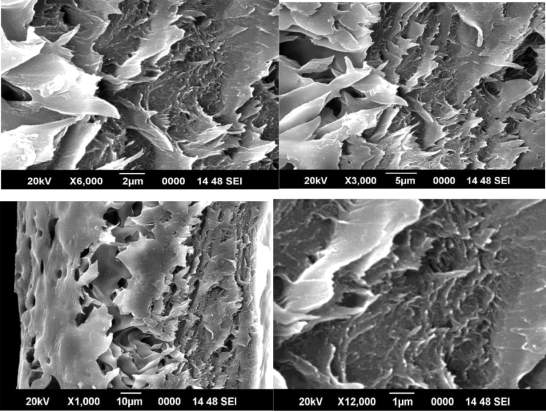
Figure 11 SEM image of 1% ZIF 8/PAI membrane
Unlike agglomerations of molecular sieve particles that have been previously reported in mixed matrix membranes prepared with other molecular sieves, the surface of these large ZIF-8 clusters as revealed in figures 11, 12 and 13 looks fairly smooth. Also, almost no defects were observed for these clusters among all the ZIF-8/PAI dense film samples. Since film samples were randomly fractured for SEM analysis, we believe that the mostly non-defective feature of these large ZIF-8 clusters shown in figures 11, 12 and 13 is representative of their interior structures. It is important to understand the formation mechanism of these large ZIF-8 clusters and their impacts on gas transport properties of the mixed matrix membrane to allow extension to practical asymmetric structures. By achieving the desired uniform distribution of individual ZIF-8 particles with the PAI matrix we can achieve outstanding gas separation results.
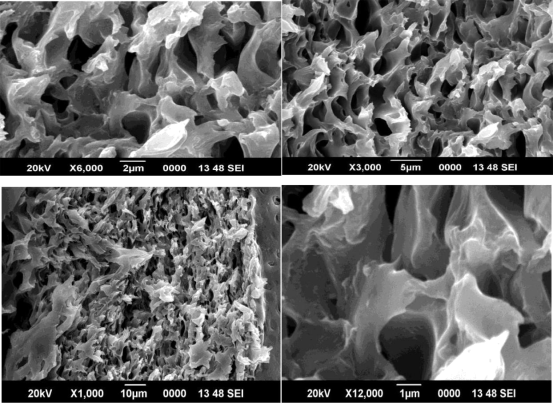
Figure 12 SEM image of 2% ZIF 8/PAI membrane
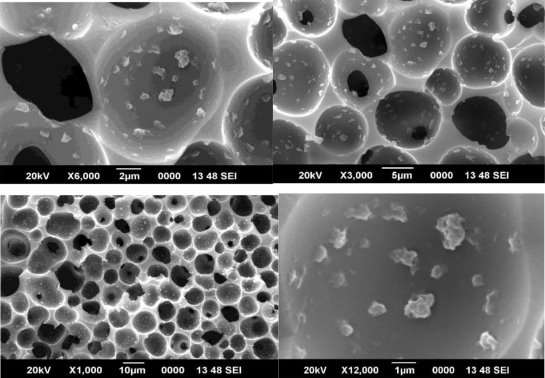
Figure 13 SEM image of 3% ZIF 8/PAI membrane
The cross sectional view of the ZIF 8/PAI membranes shows good adhesion between the inorganic filler ZIF 8 and the polymeric membrane PAI. The figures show the SEM images of 1%, 2% and 3% ZIF 8/PAI membranes prepared respectively.
FTIR Analysis of ZIF 8/PAI membranes
The FTIR results shows that the aluminosilicates are present in the ZIF 8/PAI membranes prepared. The aluminosilicates are present due to the presence of ZIF 8 nanocrystals.
|
|
FTIR Results Conjugated cyclic |
Aluminosilicates |
||||||||||||||||||||||||||||||
|
120 |
||||||||||||||||||||||||||||||||
|
100 |
||||||||||||||||||||||||||||||||
|
%T |
80 |
Unsaturated aromatic |
||||||||||||||||||||||||||||||
|
60 |
||||||||||||||||||||||||||||||||
|
40 |
||||||||||||||||||||||||||||||||
|
20 |
ketoaldehydes or enols dimer |
esters and lactones |
||||||||||||||||||||||||||||||
|
3691 |
2970 |
|||||||||||||||||||||||||||||||
|
4000 |
3897 |
3794 |
3588 |
3485 |
3382 |
3279 |
3176 |
3073 |
2867 |
2764 |
2661 |
2558 |
2455 |
2352 |
2249 |
2146 |
2043 |
1940 |
1837 |
1734 |
1631 |
1528 |
1425 |
1322 |
1219 |
1116 |
1013 |
910 |
807 |
704 |
601 |
498 |
|
cm-1 |
||||||||||||||||||||||||||||||||
|
Series1 |
Series2 |
Series3 |
||||||||||||||||||||||||||||||
FTIR Analysis of CMS/PAI membranes
The FTIR results shows that the carbon bonds are present in the CMS/PAI membranes prepared. The carbon bonds are present due to the presence of CMS particles.
|
FTIR Results |
Carbon bonds |
|||||||||||||||||||||||||||||||
|
120 |
||||||||||||||||||||||||||||||||
|
100 |
||||||||||||||||||||||||||||||||
|
%T |
80 |
|||||||||||||||||||||||||||||||
|
60 |
unsaturated aromatic |
|||||||||||||||||||||||||||||||
|
40 |
dimer |
|||||||||||||||||||||||||||||||
|
ketoaldehydes or enols |
||||||||||||||||||||||||||||||||
|
20 |
||||||||||||||||||||||||||||||||
|
Conjugated cyclic esters and lactones |
||||||||||||||||||||||||||||||||
|
3691 |
2970 |
|||||||||||||||||||||||||||||||
|
4000 |
3897 |
3794 |
3588 |
3485 |
3382 |
3279 |
3176 |
3073 |
2867 |
2764 |
2661 |
2558 |
2455 |
2352 |
2249 |
2146 |
2043 |
1940 |
1837 |
1734 |
1631 |
1528 |
1425 |
1322 |
1219 |
1116 |
1013 |
910 |
807 |
704 |
601 |
498 |
|
cm-1 |
||||||||||||||||||||||||||||||||
|
Series1 |
Series2 |
Series3 |
||||||||||||||||||||||||||||||
Material Strength of the Membranes
The material strength of the membranes prepared were studied by the performing Stress-Strain tests. The Universal Testing Machine was used to perform the tests. The samples of the membranes were cut into dimensions of height 30mm, width 10mm and thickness 0.45mm. The initial gauge length was set at 20mm. The samples were placed in a sample holder one at a time and the tests were performed. The data were recorded for respective samples.
For 3% ZIF 8/PAI membrane the Stress Vs Strain test couldn’t be performed as the membranes formed were extremely brittle in nature. Therefore it can be seen that as the concentration of inorganic fillers in the membranes increased the amount of force that the membranes could withstand kept on decreasing. But the increase in the inorganic filler concentration of the membranes increases its permeability and selectivity. Therefore optimisation must be done to determine the optimum amount of inorganic filler that has to be added to fabricate the membrane.
Table 4 Material Strength of CMS/PAI and ZIF 8/PAI membranes
|
Membrane |
Break |
Ultimate |
% Total |
Ultimate |
Yield |
Ultimate |
|
Composition |
Distance |
force (N) |
Elongation |
Stress |
Stress |
Strength |
|
(mm) |
(MPa) |
(MPa) |
(%) |
|||
|
CMS/PAI |
||||||
|
1% |
1.79 |
24.3 |
8.95 |
5.41 |
5.41 |
3.72 |
|
2% |
1.62 |
23 |
8.08 |
5.10 |
0.0444 |
4.89 |
|
3% |
2.98 |
12.1 |
14.9 |
2.69 |
0.0118 |
3.87 |
|
ZIF 8/PAI |
||||||
|
1% |
1.75 |
11.6 |
8.77 |
2.58 |
0.00741 |
8.25 |
|
2% |
0.684 |
4.43 |
3.42 |
0.985 |
0.0148 |
1.84 |
|
3% |
– |
– |
– |
– |
– |
– |
Hydrophilic Nature and Porosity Determination Test
The results of the test are tabulated below:
Table 5 Hydrophilic Nature and Porosity
|
Membranes |
% Inorganic |
Wet weight of |
Dry weight |
Amount of |
Porosity |
|
filler used |
the membrane |
of the |
water |
||
|
(g) |
membrane |
absorbed |
|||
|
(g) |
(g) |
||||
|
1 |
0.0225 |
0.0079 |
0.0146 |
0.686 |
|
|
ZIF 8/PAI |
2 |
0.0300 |
0.0105 |
0.0195 |
0.687 |
|
3 |
0.0378 |
0.0130 |
0.0248 |
0.693 |
|
|
1 |
0.0068 |
0.0055 |
0.0013 |
0.219 |
|
|
CMS/PAI |
2 |
0.0120 |
0.0100 |
0.0020 |
0.191 |
|
3 |
0.0178 |
0.0150 |
0.0028 |
0/181 |
From this test we can confirm that the membranes prepared were hydrophilic in nature and as the concentration of the inorganic fillers goes on increasing the membrane water absorption capacity also increases. The porosity of the membranes is calculated by using the formula given below:
|
( |
) / d |
||||
|
1 |
2 |
w |
|||
|
( |
) / d |
w |
2 |
/ d |
p |
|
1 |
2 |
||||

Where, Density of water dw= 1000 g/cc
Density of polymer dp= 1183 g/cc
Weight of wet polymer = w1
Weight of dry polymer = w2
CONCLUSIONS
Through this study, the following conclusion have been drawn:
The synthesis of ZIF 8 nanocrystals and CMS particles was done successfully and the various tests required to prove confirmation of these particles were also done. The ZIF 8 nanocrystals formed had a rhombic dodecahedral crystal structure and the size of the crystals were less than 100nm. The CMS particles formed where micro particles in size and showed good adhesion to the polymer matrix.
The mixed matrix membrane fabrication using these fillers was also done successfully and the various characterization tests show us that the membranes can be used for CO2/CH4 gas separation. The characterization tests show us that the CMS/PAI membranes provide high permeability and selectivity as the concentration of the inorganic fillers increased and ZIF 8/PAI membranes provide high thermal and chemical stability along with a good membrane structure as the concentration of inorganic fillers increased.
The material strength analysis show us that the CMS/PAI membrane can withstand more force and are stronger than the ZIF 8/PAI membranes. It must also be noted as the concentration of inorganic fillers in the membranes increased the amount of force that the membranes could withstand kept on decreasing. But the increase in the inorganic filler concentration of the membranes increases its permeability and selectivity. Therefore optimisation must be done to determine the optimum amount of inorganic filler that has to be added to fabricate the membrane.
Optimisation was done and it was found that the 2% CMS/PAI membranes was found to be best suitable for the given CO2/CH4 gas separation The various tests for permeation, permeability and selectivity is to be done for ZIF 8/PAI and CMS/PAI membranes. The performance of both the membranes is to be compared and the suitable membrane will be used for CO2/CH4 separation.
REFERENCES
- Xinlei Liu, Yanshuo Li, Yujie Ban, Yuan Peng, Hua Jin, Helge Bux, Longya Xu, Jürgen Caro and Weishen Yang, Supplementary Information Improvement ofhydrothermal stability of zeolitic imidazolate frameworks, The Royal Society of Chemistry 2013
- Tai-Shung Chung, Lan Ying Jiang, Yi Lia, Santi Kulprathipanja, Mixed matrixmembranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation, Prog. Polym. Sci. 32 (2007) 483-507
- Janosch Cravillon, Roman Nayuk, Sergej Springer, Armin Feldhoff, Klaus Huber and Michael Wiebcke, Controlling Zeolitic Imidazolate Framework Nano- andMicrocrystal Formation: Insight into Crystal Growth by Time-Resolved In Situ Static Light Scattering, Chemistry of Materials
- Yichang Pan, Yunyang Liu, Gaofeng Zeng, Lan Zhao and Zhiping Lai, Rapidsynthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system,
Chem. Commun., 2011,47, 2071-2073
- De Q. Vu and William J. Koros, Stephen J. Miller, High Pressure CO2/CH4SeparationUsing Carbon Molecular Sieve Hollow Fiber Membranes, Ind. Eng. Chem. Res. 2002, 41, 367-380
- De Q. Vu, William J. Koros, Stephen J. Miller, Mixed matrix membranes using carbonmolecular sieves I. Preparation and experimental results, Journal of Membrane Science 211 (2003) 311-334
- De Q. Vu, William J. Koros, Stephen J. Miller, Mixed matrix membranes using carbonmolecular sieves II. Modeling permeation behaviour, Journal of Membrane Science 211 (2003) 335-348
- De Q. Vu, William J. Koros, Stephen J. Miller, Effect of condensable impurity inCO2/CH4 gas feeds on performance of mixed matrix membranes using carbon molecular sieves, Journal of Membrane Science 221 (2003) 233-239
- Chen Zhang, Ying Dai, Justin R. Johnson, Oguz Karvan, William J. Koros, Highperformance ZIF-8/6FDA-DAM mixed matrix membrane for propylene/propane separations, Journal of Membrane Science 389 (2012) 34- 42
- Yuan Zhang, Jaka Sunarsoc, Shaomin Liu, Rong Wang, Current status anddevelopment of membranes for CO2/CH4 separation: A review, International Journal of Greenhouse Gas Control 12(2013) 84-107
- Chen Zhang, Kuang Zhang, Liren Xu, Ying Labreche, Brian Kraftschik, and William J. Koros, Highly Scalable ZIF-Based Mixed-Matrix Hollow Fiber Membranes forAdvanced Hydrocarbon Separations, DOI 10.1002/aic.14496, Published online May 29, 2014 in Wiley Online Library (wileyonlinelibrary.com)
- Xinlei Liu, Yanshuo Li, Yujie Ban, Yuan Peng, Hua Jin, Helge Bux, Longya Xu, Jürgen Caro and Weishen Yang, Supplementary Information Improvement ofhydrothermal stability of zeolitic imidazolate frameworks, The Royal Society of Chemistry 2013
- M.A. Aroon, A.F. Ismail, T. Matsuura, M.M. Montazer-Rahmati, Performance studiesof mixed matrix membranes for gas separation: A review, Separation and Purification Technology 75(2010) 229-242
- P.S. Goh, A.F. Ismail, S.M. Sanip, B.C. Ng, M. Aziz, Recent advances of inorganic fillersin mixed matrix membrane for gas separation, Separation and Purification Technology 81 (2011) 243-264
- Asim Laeeq Khan, Sreeprasanth P. Sree, Johan A Martens, Muhammad Tayub Raza, Ivo F.J. Vankelecom, Mixed matrix membranes comprising of matrimid and mesoporousCOK-12: preparation and gas separation properties, Journal of Membrane Science
- Boor Singh Lalia, Victor Kochkodan, Raed Hashaikeh, Nidal Hilal, A review onmembrane fabrication: Structure, properties and performance relationship, Desalination 326 (2013) 77-95
- Lloyd M. Robeson, The upper bound revisited,Journal of Membrane Science 320 (2008)390-400
- Kanna Aoki, Katsuki Kusakabe, Shigeharu Morooka, Gas permeation properties of A-type zeolite membrane formed on porous substrate by hydrothermal synthesis, Journal of Membrane Science 141(1998) 197±205
- P. Bernardo, E. Drioli, and G. Golemme, Membrane Gas Separation: A Review/State ofthe Art, Ind. Eng. Chem. Res. 2009, 48, 4638-4663
- Juergen Caro, Manfred Noack, Zeolite membranes-Recent developments and progress, Microporous and Mesoporous Materials 115(2008) 215-233
- Dominic T. Clausi, William J. Koros, Formation of defect-free polyimide hollow fibermembranes for gas separations, Journal of Membrane Science 167 (2000) 79-89
- Fatereh Dorosti, Mohammadreza Omidkhah, Reza Abedini,Fabrication andcharacterization of Matrimid/MIL-53 mixed matrix membrane for CO2/CH4 separation, chemical engineering research and design
- Hans H. Funke, Andrew M. Argo, John L. Falconer, and Richard D. Noble,Separations of Cyclic, Branched, and Linear Hydrocarbon Mixtures through Silicalite Membranes, Ind. Eng. Chem. Res. 1997, 36, 137-143
- A.F. Ismail, P.S. Goh, S.M. Sanip, M. Aziz, Transport and separ