Heterocycles as Triazine Derivatives
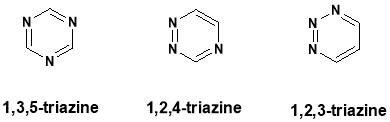 Heterocycles are ring with nitrogen practical called pyridine, pyrimidine, etc., carbon containg ring called benzene. There are so many compounds are which are belonging to heterocycles. One of them is triazine. Triazine is a six mambered heterocyclic ring system. It is similar to benzene ring system. Triazine has three isomers which single alternative. They are known as 1,2,3-triazine, 1,2,4-triazine and 1,3,5-triazine.
Heterocycles are ring with nitrogen practical called pyridine, pyrimidine, etc., carbon containg ring called benzene. There are so many compounds are which are belonging to heterocycles. One of them is triazine. Triazine is a six mambered heterocyclic ring system. It is similar to benzene ring system. Triazine has three isomers which single alternative. They are known as 1,2,3-triazine, 1,2,4-triazine and 1,3,5-triazine.
Antimalerial and other diseases are very common in our society and all area. Currently antimicrobial agents are not as much effective due to change of environmental progress in hygienic way. So in that way we improve our method and effort to produce and make new and unknown antimicrobial agents. Such a certain chemicals in the environment can cause endocrine disruption in exposed humans and wildlife. The triazine derivatives1-6 has been associated with wide derivatives of therapeutic activities7-12 such as antibacterial29 and antimalarial. The quest for a more reliable and suitable drugs are always fascinating and challengeable. A many derivatives drugs containing simple heterocyclic moieties that have been use these days13.
Triazine derivatives have been widely studied in their synthetic methodologies and reactivity in many activities like antagonists14, age related macular degeneration15, analgesic18, anticancer activity19-20, anticonvulsant activity21, antimicrobial activity22, anxioselective agents16, muscle relaxant activity17 and antiinflammatory activities23.
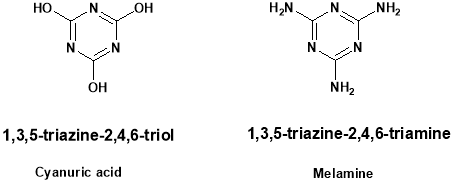
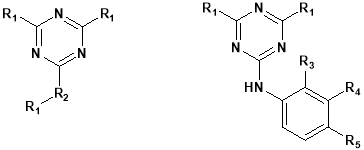
Triazine chemistry was adapted for educational application in development of simazine, a widely used herbicide. The laboratory was designed to faster a sense of the applications of chemistry in the world. The modification of herbicide remediation has been accomplished using triazine chemistry. Triazine is thermally stable unless heated to above 6000 C. The triazine ring is resistant to electrophilic substitution. It may undergo ring cleavage with nucleophiles. The most common triazine derivatives are shown below.
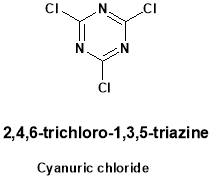
Development of valuable methods and reaction for the preparation of many triazine is still challengeable. The main issues in modern synthetic organic chemistry are mildness, improvement of efficiency and selectivity of toxic reagents and by-products. Triazine derivatives represent an important class of complex compound due to their biological activity. They are known to anti-protozoals, estrogen receptor modulators, cyclindependent kinase odulators and antimicrobials. Triazines are a class of compounds well known for a long time continues the object of considerable interest mainly due to their use in different fields and polymer photo stabilizers.
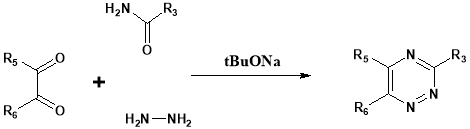
In general amides like benzamide and formamide when condensed with aromatic ketones formed a jelly mass. That jelly mass is the condensed product, it can be cyclised to 1,2,4-substituted triazines by reaction with hydrazine hydrate24.
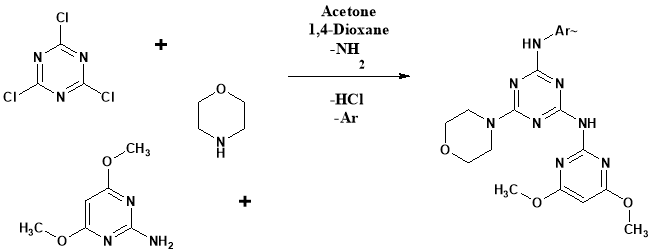
Parikh Kalpesh S. et al prepared various s-triazine derivatives25. 4, 6-dimethoxypyrimidin-2-aminecondensed with trichloro s-triazine. The reaction mixture react with 8-Hydroxy quinoline. Finally various aromatic amines derivatives were allowed to react and got the product. Anti bacterial and antifungal study has been performed.
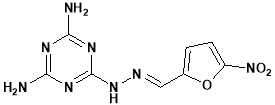 Klenke Bukhard26 et al have reported Polyamine biosynthesis and function has been shown to be a good drug target in some parasitic protozoa and it is proposed that the pathway might also represent a target in the malaria parasite Plasmodium falciparum.
Klenke Bukhard26 et al have reported Polyamine biosynthesis and function has been shown to be a good drug target in some parasitic protozoa and it is proposed that the pathway might also represent a target in the malaria parasite Plasmodium falciparum.
A series of 1,3,5-triazine-substituted polyamine analogues were tested for activity against Plasmodium falciparum in vitro The series showed activity against the parasites and were generally more active against the chloroquine-resistant line K1 than the chloroquine-susceptible line NF54. Addition of multiple triazine units in general led to increased activity of the compounds.
Malamine is called 1,3,5-triazine. It is applied as a part of collecting of gums. Chloro substituted derivatives are applied for eagerness responsive colors. Mixture of water and triazine involved H2S from fixed gas. Melamine is most often reacted with formaldehyde for for the production of resins27. The polymers formed from melamine formaldehyde resins have excellent chemical preoperties.
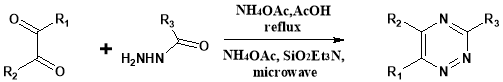
Z Zhao et al28. reported the new scope of triazine synthesis by the application of microwave technology, shown in below reaction:
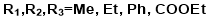
Akshay Desai30 made 2,4,6-trisubstituted triazine derivatives. Also give antibacterial and antifungal activity in his paper, to show the importance of triazine in medicinal and in other chemical research production. Reaction is shown below:
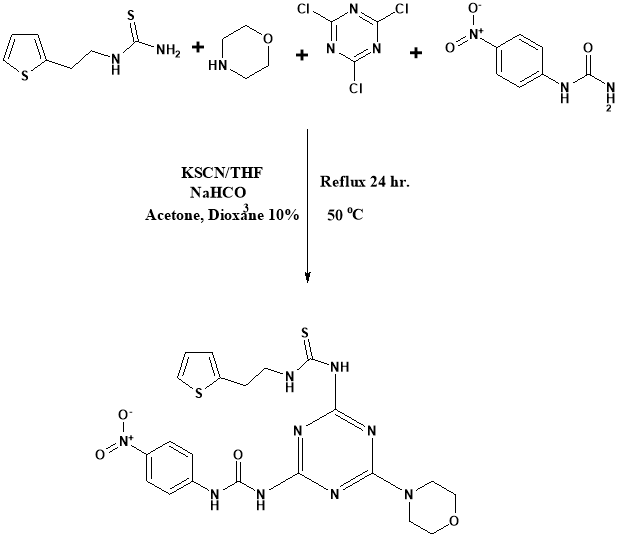
Long-chih Hwang43 made fused 1,2,4-triazine. Derivative triazine have acquired considerable importance because of their CNS depressant, anti-allergy, anti-inflammatory and antimicrobial properties31-34. However 3-substituted derivatives of triazine revealing their anti-HIV and anti-cancer activities35-42.
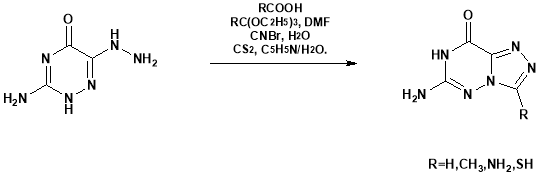
Bhawsar S. B. et al44 synthesized a new derivatives of triazine 2, 4-bisarylamino-6-(2′-(2”-amino-6”- arylpyrimidin-4”-yl)aryloxy) .
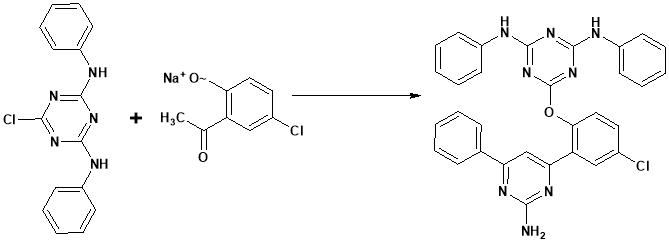
Ravikumar et al45 prepared and showed the 3D-QSAR and molecular docking studies of 1, 3,5-triazine-2, 4-diamine derivatives against r-RNA. The obtaining product is reported it as a promising antibacterial translational inhibitor.
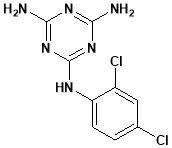
Irsogladine is commonly used in Japan46 as anti-gastric ulcer agent.
- Other pharmacological activities exhibited by 1,2,3-Triazine derivatives are enlisted below:
- African sleeping sickness and syphilis
- Antiarthritic
- Histamine shocked guinea pigs
- Local anesthetic
- Anticonvulsant
- Antiviral
- Insecticidal
- Muscle relaxant
- Estrogen receptor modulators
- Cyclindependent kinase modulators
- Antitumor
- Tubercular
- Diuretic
- Antiprotozoal
Further literature survey reveals that s-triazine derivatives coupled with some Pyrimidinone derivatives as powerful antitubercular agents which never such derivatives in research of better useful biological molecules. Further reports says that s-triazine derivatives have also diverse biological potential.
- Serullas A. Ann. Chim Phys.; 38, 379, 1828.
- Liebig J. Pogg. Ann. 15, 359, 1829.
- National Committee for Clinical Laboratory Standard. Reference method for broth dilution antifungal susceptibility testing of yeasts Approved standard M27A. 1997, NCCLS, Wayne, PA.
- Banks C. K., Gruhza O.M., Tillitson E.W., John controulis. J Am Chem Soc; 66;1771, 1944.
- Zollinger H, Angew Chem; 73, 132, 1961.
- Dudley J R., J Am Chem Soc, 73, 2990, 1951.
- Dighade S R, Patil S D, Chincholkar M M & Dighade N R, Asian J Chem, 15(2), 2003,1184.
- Anjani Solankee &Indrajit Thakor, Ind J of Chem; 45B, 517, 2006.
- Solanki A and Patel J; Ind J of Chem, Vol. 43B; pp.1580, 2004.
- Desai K. R., Patel R.B., Desai P.S., Chikhalia K.H..; J Ind Chem Soc.;Vol.80; Pp-138, 2003.
- Curd F H S; J ChemSoc, 343, 1946.
- Lanalia N A, Thaker K A; J Ind Chem Soc, 59(9),1099-1101, 1982.
- Baldaniya B. B. and Patel P. K., Synthesis Antibacterial and Antifungal Activities of s-Triazine Derivatives. E- Journal of Chemistry.; 6(3): 673-680, 2009.
- Settimo F.D. and Primofiore G. 3-Aryl [1, 2, 4] triazino [4, 3-a] benzimidazol-4(10H)-ones: A new class of selective A1 adenosine receptor antagonists. J. Med.Chem.; 44, 316-327, 2001.
- Palanki M. S. S. and Akiyama H. Development of prodrug 4-chloro-3-(5-methyl-3-{[4-(2-pyrrolidin-1-ylethoxy) phenyl] amino} 1, 2, 4-benzotriazin-7-yl) phenyl benzoate (TG!100801): A topically administered therapeutic candidate in clinical trials for the treatment of age-related macular degeneration. J. Med.Chem. 51, 1546-1559, 2008;.
- MakhloufAbdelmoneim A. and MakladYousreya A. Synthesis and analgesic- antiinflammatory activities of some 1, 2, 4-triazine derivatives, Arzneimittel-Forschung,; 54(1): 42-49, 2004.
- El-Gendy Z. and Morsy J. M. Synthesis of heterobicyclic nitrogen systems bearing a 1,2,4-triazine moiety as anticancer drugs: part iv. Phosphorus, Sulfur, and Silicon; 178: 2055-2071, 2003.
- Sztanke K. and Pasternak K. Synthesis, structure elucidation and identification of antitumoural properties of novel fused 1,2,4-triazine aryl derivatives. Europ. J. of medi. Chem,; 43: 1085-1094, 2008.
- Mallikarjuna B. P. and Suresh Kumar G. V. Synthesis and anticonvulsant activity of some potent 5,6-bis aryl 1,2,4-triazines. J. Zhejiang UnivSci B. 8(7): 526-532, 2007;.
- Modzelewska-Banachiewicz B. and Kowalski C. Biological activity of 1,2,4-triazineand 1,2,4-triazole derivatives., Annalesuniversitatismariae curie – skÅ‚odowskalublin – polonia. 2007;LXII.
- Sztanke K. and Fidecka S. Antinociceptive activity of new imidazolidine carbonyl derivatives. Part 4. synthesis and pharmacological activity of 8-aryl-3,4-dioxo-2H,8H-6,7-dihydroimidazo[2,1-c][1,2,4]triazines. Europ. J. of Medi. Chem.; 40: 127-134, 2005.
- Costanzo A, Guerrini G, Benzodiazepine receptor ligands.7. Synthesis and pharmacological evaluation of new 3-esters of the 8-chloropyrazolo[5,1-c] [1,2,4]benzotriazine 5-oxide. 3-(2- Thienylmethoxycarbonyl) derivative: an anxioselective agent in rodents., Journal Med Chem., 45(26), 5710-20, 2002.
- Hunt J T and Mitt T, Discovery of the pyrrolo[2,1-f][1,2,4]Triazine nucleus as a new Kinase inhibitor template., Journal Med.Chem., , 47, 4054-4059, 2004.
- T. Phucho, A. Nongpiur, S. Tumtin, R. Nongrum, B. Myrboh, and R. L. Nongkhlaw.,Novel one pot synthesis of substituted 1,2,4-triazines., Arkat USA Inc., ARKIVOC- 2008 (xv) 79-87.
- Kalpesh S. Parikh, Sandip P.Vyas, Design, characterization and biological evaluation of a new series of s-triazines derived with quinolines, Scholar Research Library, 4(3), 1359-1362, 2012.
- Burkhard Klenke,, Michael P. Barrett, Reto Brun and Ian H. Gilbert, Antiplasmodial activity of a series of 1,3,5-triazine-substituted polyamines, Journal of Antimicrobial Chemotherapy, 52, 290-293, 2003.
- Diem, H.; Matthias, G., Amino Resins. In Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; Wiley-VCH: Weinheim, Germany, 2006.
- Z. Zhao, W. H. Leister, K. A. Strauss, D. D. Wisnoski, and C. W. Lindsley; Tetrahedron Lett. 44, 1123-1127, 2003.
- Baldaniya B. B. and Patel P K, Synthesis Antibacterial and Antifungal Activities of s-Triazine Derivatives. E- Journal of Chemistry, 6(3): 673-680, 2009;.
- Akshay D. Desai, Dharmesh H. Mahajan, and Kishor H Chikhalia, synthesis of novel aliphatic thiourea derivatives containing s-triazine moiety as potential antimicrobial agents, Indian journal of chemistry, vol-46B, 1169-1173, july-2007.
- Deshmukh, A. A. Mody, M. K. Ramalingam, T. Sattur, P. B. Indian J. Chem., 23B, 793, 1984.
- Loye, B. Musser, J. H. Brown, R. E.; Jones, H. Kahenan, R. Huang, F. C. Khandwala, A. Sonnio-Goldman, P. Leibowitz, M. J. J. Med. Chem., 28, 363, 1985.
- Gupta, R. Gupta, A. K. Paul, S. Kachroo, P. L. Indian J. Chem., 37B, 1211, 1998.
- Hiremath, S. P. Ullagaddi, A. Shivaramayya, K. Purohit, M.G. Indian J. Heterocycl. Chem., 3, 145, 1999.
- Pomarnacka, E. Acta Pol. Pharm. 55, 481, 1998,.
- Abdel-Rahman, R. M. Morsy, J. M. Hanafy, F. Amene, H. A. Pharmazie, 54, 347, 1999.
- Abdel-Rahman, R. M. Morsy, J. M. El-Edfawy, S. Amine, H. A. Pharmazie , 54, 667, 1999.
- Abdel-Rahman, R. M. Pharmazie, 56, 18, 2001.
- Abdel-Rahman, R. M. Pharmazie, 56, 275, 2001.
- El-Gendy, Z. Morsy, J. M. Allimony, H. A. Ali, W. R. Abdel-Rahman, R. M. Pharmazie,56, 376, 2001.
- Holla, B. S. Rao, B. S. Gonsalves, R. Sarojini, B. K. Shridhara, K. Farmaco, 57, 693, 2002.
- Habib, N. S. Soliman, R. Ismail, K. Hassan, A. M. Sarg, M. T. Boll Chim Farm., 142,396, 2003.
- Long-chih Hwang, Chun-Hsien Tu, Jung-Hui Wang and Gene-Hsiang Lee, synthesis and molecular structure of 6-Amino-3-benzylmercapto-1,2,4-triazolo[3,4-f][1,2,4]triazin-8(7H)-one,; Molecules, 11, 169-176, 2006.
- Bhawasar, S. B. Shinde, D. B. Mane, D. V. Thore, S. N. Gajare, A. S. Shingare, M. S.; Pol. J. Chem., 70(6), 809-812, 1996.
- Ravikumar, M. Mahmood, S. K. Sivakumari, N. .J of Mole Graph and Model , 26(8), 1338-1352, 2008.
- Saczewski, F. Bulakowska, A. Bednarski, P. Grunert, R. Eur. J. Med. Chem., 41, 219-225, 2006.