History and Properties of Rubber
History of Rubber
Rubber was first discovered by the Indians living in Central and South America at around 1000 CE. The native people of the Americas used latex from the rubber trees (which they called it the cahuchu tree) and learned how to make waterproof clothes and footwears.1 They also used the latex to make bouncing balls for ball games.1 In 1736, a French explorer named Charles Marie de La Condamine travelled to Ecuador and discovered the Hevea tree (which is known as the rubber tree), he was the first European to have discovered rubber. He sent samples of this rubber to the French Academy of Sciences, this prompted their interest of rubber.2

Figure 1: Latex liquid collected from the Hevea rubber tree 3
In 1770, English engineer named Edward Nairne created several rubber cubes and discovered that they had erasing properties. This meant that they can be able to erase pencil marks, this was later confirmed by English scientist named Joseph Priestley.4 In 1768, a French Chemist named Pierre Macquer studied the properties of the rubber found in South America. He found that when rubber is dissolved in ether, flexible tubes can be produced 5, this has led to rubber being an important material to many everyday applications. In 1791, a British shoemaker named Samuel Peal developed a method for waterproofing cloth, this was done by mixing turpentine (which is an oiled that is attained by the distillation of resin from pine trees) with rubber.6 In 1818, a Scottish surgeon named James Syme manufactured raincoats from waterproof cloth made from rubber.7 This was then later developed by Scottish chemist named Charles Macintosh. In 1823, he developed a method of making better waterproof raincoats by dissolving rubber with naphtha (which is an oil attained by the distillation of coal tar) and then placing it with another cloth (in order to improve the thickness of the coat). The waterproof coat became known as the Mackintosh coat.8

Figure 2: The Mackintosh Coat 9
In 1832, the first rubber factory was built, it was known as the Rosburg factory. Regrettably, when the rubber products made from the factory were left in the cold, it made them brittle, and when they were left in the sun the rubber melted, leaving customers to be discouraged.8 For many years, scientists have tried to develop new methods of increasing the strength of rubbers without being successful. However, in 1839, an American chemist named Charles Goodyear had accidently discovered a new way of making rubbers more durable. What he done was he accidentally dropped some mixture of natural rubber and sulphur on a hot stove, this has led to the vulcanization of the rubber. 8
In 1845, a Scottish inventor named Robert William Thompson invented the pneumatic tyre, this consisted of an inner belt made from natural rubber that was inflated with air, this was a major development to the motor industry because heavy steam engines could be able to travel on the roads without damaging the surface, this was further developed in 1869 where solid and hollow rubber were used to make the tyres. In the 1850s, rubber toys were being produced. 8 In 1876, an English explorer named Henry Wickham smuggled several seeds from the Hevea brasiliensis (which is the rubber tree found in Brazil) and took it to England. The English exported these seeds around the world, this has led to the increase in the supply of natural rubber around the world. 10
In 1883, an American chemist named George Oenslager developed a new method of accelerating the vulcanization of natural rubber with sulphur.7 He converted aniline into thiocarbanilide because it was easier to handle. From several experiments he conducted, he found out that thiocarbanilide was a good rubber accelerator for the vulcanization process. He was the first person to use carbon black as a filler for the rubber because it increased the strength of the rubber. 11 In 1909, a German scientist named Fritz Hofmann and his fellow scientists produced the first synthetic rubber known as methyl isoprene however the problem with this rubber is that it was expensive to make.12
In 1930, an American scientist named Wallace Carothers and his team produced a compound called chloropropene which then polymerised to form a solid which had a rubbery texture. The team had found out that the properties of this new polymer was similar to that of natural rubber. This polymer was named as Neoprene and was the first commercially successful synthetic rubber.13 Also in the 1930s, a German chemist named Walter bock had developed a new synthetic rubber which had better properties than that of natural rubber. He first tried to copolymerise dimethyl butadiene with isoprene and butadiene, this was successful as the new polymer had similar properties to the natural rubber. He then replaced dimethyl butadiene with styrene and copolymerised with butadiene, the polymer was named as SBR (styrene-butadiene rubber). This polymer was better than natural rubber because it did not wear out quicker than its counterpart, the polymer was later known as Buna-S for commercial purposes. 14 A better synthetic rubber was later developed by copolymerising acrylonitrile with butadiene, the polymer was known as NBR (Nitrile-butadiene rubber), it was the later known as Buna-N for commercial purposes.
In 1940, an American inventor named Waldon Semon produced a new synthetic rubber known as Ameripol, this was made by copolymerising butadiene with methyl methacrylate. This synthetic rubber was cheap and easy to make, so it was a no-brainer that it was used in World War 2, and helped out rubber companies such as the Goodyear Tyre and Rubber Company during the war. After the war, the need for natural rubber died down, and the need for synthetic rubbers increased dramatically.15 In the 1960s, EPDM (which is known as Ethylene propylene Terpolymer Rubber) was produced. The rubber is made by copolymerising by a diene derivative, ethylene and propylene. The rubber has many advantageous properties over the synthetic rubbers, one of them is that they have good electrical insulating properties. EPDM rubber are now found in our everyday life such as hose, solar panels, electrical insulation etc.16 The use of synthetic rubber is on a steep rise, and many new rubber materials have been made during the 21st Century.
Natural Rubber
How it is made?
Natural rubber is produced by biosynthetic processes (in the form of latex which is a white liquid that is found when you cut the plant up) in many plants, but mainly from the Hevea Brasiliensis (The rubber tree coming from Brazil). There are two biosynthetic process to make latex. In the first stage, Acetyl-coenzyme A is converted to melavanic acid which then is then converted to iso-pentyl-pyrophosphate. The second process is the polymerisation induced by the first stage of the biosynthesis, from this process latex is created. The rubber form of the latex is then form by coagulating the latex particles. The rubber is made from a polymerisation of naturally occurring cis-polyisoprene. 17
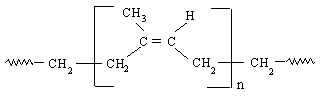
Figure 3:Cis-polyisoprene, natural rubber 18
Properties of Raw Natural Rubber
The rubber has a clear colour and hasn’t got a well-defined shape and it is soft and sticky, however when the rubber is cooled down it crystalizes, so has a well-defined shape. The rubber has a low tensile strength meaning it tends to break if a low amount of tensile stress is put into it. It has a low abrasion resistance meaning the rubber will wear out quickly. It is soluble to organic solvents such as turpentine (which is an oiled that is attained by the distillation of resin from pine trees), ether, petrol, carbon tetrachloride, and naphtha (which is an oil attained by the distillation of coal tar). It is insoluble to hydrophilic solvents such as water, acetone, and alcohol. The rubber has a high elastic property, this means that when the rubber is stretched out it gets bigger, but when it is released the rubber goes back into its original shape. The rubber can only be elastic between 10 C to 60 C, this means under hot conditions the rubber would lose its elasticity. Another property of the natural rubber is that it absorbs a large amount of water this means that it will be always wet and would need to dry it out before the next stage of the process. 19
Advantage of Raw Natural Rubber
The hardness of the rubber can easily be adjusted, so can be able to shape or coat any form of objects this is because the rubber has a high elastic property, this means that when the rubber is stretched out it gets bigger, but when it is released the rubber goes back into its original shape. It can be used as an electrical conductor or an electrical insulator. Can be able to absorb vibration and noise. It is insoluble to hydrophilic solvents such as water, acetone, and alcohol. It also has a good surface friction so does not slide about easily.
Disadvantage of Raw Natural Rubber
The rubber can only be elastic between 10 C to 60 C, this means under hot conditions the rubber would lose its elasticity. The rubber has a low tensile strength meaning it tends to break if a low amount of tensile stress is put into it. It has a low abrasion resistance meaning the rubber will wear out quickly. It has a poor resistance to any organic compounds this is because It is soluble to organic solvents such as turpentine (which is an oiled that is attained by the distillation of resin from pine trees), ether, petrol, carbon tetrachloride, and naphtha (which is an oil attained by the distillation of coal tar). Another disadvantage of natural rubber is that it has a poor resistance to ozone meaning that when the ozone reacts with the double bonds it breaks, this causes a crack.20 The crack then grows steadily because of further ozone attacks so this causes the strength of the rubber to decrease massively and cause it to break. Another disadvantage of natural rubber is it has a poor UV resistance this also means that when it reacts with the double bond it breaks; this also means that cracks will from causing the tensile strength to be severely reduced meaning the strength of the rubber would be reduced massively and causing it to break. 20 Another disadvantage is that vulcanization of the rubber can occur spontaneously, so it is hard to control the raw state of the natural rubber.
The vulcanization of Natural Rubber
To improve the properties of the natural rubber, the process of vulcanisation is used to do this. Vulcanization is a process that involves adding natural rubber to a curing agent such as sulphur. The process is done under heat, so by heating the rubber in the presence of the curing agent the physical and chemical properties of the rubber would be significantly improved. The reason it does is, because when heating the rubber in the presence of the curing agent, the polymer chains are cross-linked by the agent, therefore the free-flowing macromolecules of the polymer chains becomes more rigid.21 Vulcanization must be under controlled condition to avoid creating a massive amount of cross-linking, this means that it will avoid making the rubber less elastic and more brittle.
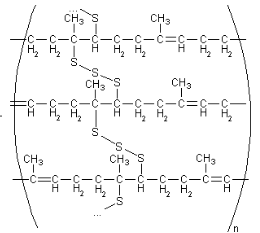
Figure 4: Cis-polyisoprene with sulphur cross-links after vulcanization 23
Properties of Vulcanised Rubber
The rubber has a clear colour and it is hard and not sticky, when the rubber is cooled down it crystalizes, so has a well-defined shape. The rubber has a high tensile strength meaning it does not tend to break if a low amount of tensile stress is put into it. It has a high abrasion resistance meaning the rubber will not wear out quickly. It is not soluble to organic solvents such as turpentine (which is an oiled that is attained by the distillation of resin from pine trees), ether, petrol, carbon tetrachloride, and naphtha (which is an oil attained by the distillation of coal tar). It is insoluble to hydrophilic solvents such as water, acetone, and alcohol. The rubber has a high elastic property, this means that when the rubber is stretched out it gets bigger, but when it is released the rubber goes back into its original shape. The rubber can only be elastic between -40 C to 100 C which is better than what the given temperature range is for the natural rubber in its raw state, this means under hot conditions the rubber would not lose its elasticity. Another property of the natural rubber is that it does not absorb a large amount of water this means it can be dried easily before the next stage of the process. 23
Advantages of Vulcanised Rubber
The hardness of the rubber can easily be adjusted, so can be able to shape or coat any form of objects this is because the rubber has a high elastic property, this means that when the rubber is stretched out it gets bigger, but when it is released the rubber goes back into its original shape. It can be used as an electrical conductor or an electrical insulator. Can be able to absorb vibration and noise. It is insoluble to hydrophilic solvents such as water, acetone, and alcohol. It is also insoluble to organic solvents such as turpentine (which is an oiled that is attained by the distillation of resin from pine trees), ether, petrol, carbon tetrachloride, and naphtha (which is an oil attained by the distillation of coal tar). It also has a good surface friction so does not slide about easily. The rubber has a high tensile strength meaning it does not tend to break if a low amount of tensile stress is put into it. It has a high abrasion resistance meaning the rubber will not wear out quickly. The rubber can only be elastic between -40 C to 100 C which is better than what the given temperature range is for the natural rubber in its raw state, this means under hot conditions the rubber would not lose its elasticity. Another advantage of the vulcanized rubber is that it does not absorb a large amount of water this means it can be dried easily before the next stage of the process. It has a better resistance to ozone and UV this means that less cracks form than in its raw state. This means that it is less likely to break than the raw natural rubber.
Disadvantages of Vulcanised Rubber
If the vulcanized rubber is burned it can be toxic for the workers and consumers this is because it contains sulphur cross-links which can form sulphur dioxide. Vulcanization must be under controlled condition to avoid creating a massive amount of cross-linking, this means that it will avoid making the rubber less elastic and more brittle. Another disadvantage of vulcanized rubber, is that when you heat the rubber it melts and then cools quickly, this is quite dangerous for the people who are handling it. Another disadvantage of using vulcanized rubber, is natural rubber are becoming more scarce, therefore the cost of making the vulcanized rubber is increasing. To overcome this problem, scientist have been trying to make vulcanized rubber out of synthetic rubbers such as styrene-butadiene rubbers (SBR), however the problem with this is that it is not cost effect and it is very expensive to make.24
Applications of Natural Rubber and Vulcanized Rubber
The main usage of natural rubber is in the motor industry. It is used to make tyres and tubes in vehicles this is because it decreases any generation of heat in the tyres. It also offers high mechanical resistance. Tyres and tubes are used mainly in heavy duty vehicles such as trucks and tractors. 23 Natural rubbers can be used to make toys, footwear, balloons, glue and condoms. Another important application of natural rubbers is that they can be used to make latex gloves. Latex gloves are used in many industries such as the medical industry, chemical industry, and engineering industry, this is because due to the chemical and physical properties of the natural rubber mentioned above it can protect workers and consumers’ hands from hazardous chemicals.25
There are several applications for the vulcanized rubber. Vulcanized rubbers are much better to produce tyres than natural rubber this is due to having high abrasion resistance meaning tyres won’t wear out quickly. The rubber is flexible, this means that it can be used to make hoses, tubes, coats etc… Shock absorbers in vehicles are made from vulcanized rubbers due to being able to absorb vibrations easily.26 As the rubber, does not dissolve in water, it can be used to make waterproof clothing and footwears. Another application of using vulcanized rubber is that they can be used to produce cables for telephone housing, and can be able to produce insulations and conductors for electrical instruments. The reason why they are used in electrical instruments, is that have good electrical insulating and conducting properties.
Synthetic Rubbers
Synthetic Rubbers are made by copolymerising two different monomers under certain conditions. There are 3 conditions (the mixture can be in) it can be done in such as: emulsion, suspension and solution. There are 9 major classes of synthetic rubbers: Styrene-butadiene rubber (SBR), Poly(Butadiene-acrylonitrile) rubbers (NBR), Butyl Rubbers (IIR), Polychloroprene (CR), Ethylene-propylene rubbers (EPDM), Urethane rubbers (EU), Silicone rubbers (VMQ), Fluoroelestomer (FKM), Flurosilicone (FVMQ).27 In this 4 major classes are being discussed.
Polychloroprene
In 1930, an American scientist named Wallace Carothers and his team produced a compound called chloropropene which then polymerised to form a solid which had a rubbery texture. The team had found out that the properties of this new polymer was similar to that of natural rubber. This polymer was named as Neoprene and was the first commercially successful synthetic rubber.13
Neoprene is produced by the free-radical polymerisation of chloroprene. The chloroprene undergoes polymerisation under aqueous emulsion.28
Properties of Polychloroprene
The rubber has a greyish green colour.28 The rubber has a high tensile strength meaning it does not tend to break if a low amount of tensile stress is put into it. It has a high abrasion resistance meaning the rubber will not wear out quickly. It can dissolve with organic solvents such as turpentine (which is an oiled that is attained by the distillation of resin from pine trees), ether, petrol, carbon tetrachloride, and naphtha (which is an oil attained by the distillation of coal tar). It is insoluble to hydrophilic solvents such as water, acetone, and alcohol. The rubber has a moderate elastic property, this means that when the rubber is stretched out it gets bigger, but when it is released the rubber goes back into its original shape. The rubber can only be elastic between -34 C to 100 C which is better than what the given temperature range is for the natural rubber, this means under hot conditions the rubber would not lose its elasticity. The rubber has a low flammability, and has a high resistance to weather and ozone.29
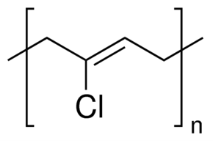
Figure 5: Polychloroprene 30
Advantages of Polychloroprene
This rubber is tougher than natural rubber because it has a higher tensile strength than natural rubber meaning it does not tend to break if a low amount of tensile stress is put into it. It has a very high resistance to hot conditions and other weather conditions; this means that it does not melt or break easily. Another advantage of polychloroprene rubber is that they have a better resistance to ozone and UV this means that less cracks forms than in its natural counterpart. This means that it is less likely to break than the natural rubber. It has a low flammability and can be able to self-extinguish if it is on fire. The structure of the polychloroprene can be altered to create a new compound which can exhibit different chemical and physical properties.
Disadvantages of Polychloroprene
The rubber has a poor resistance to aromatic compounds such as benzene derivatives, they also have poor resistance to carbonyl compounds such as ketones and esters, and, also has a poor resistance to strong oxidising agents. Another disadvantage of polychloroprene, is that they are quite expensive to produce. 29
Typical applications of Polychloroprene
- Production of hoses
- Making belts
- Making cable covers for electrical instruments
Styrene-butadiene Rubber
In the 1930s, a German chemist named Walter bock had developed a new synthetic rubber which had better properties than that of natural rubber. He first tried to copolymerise dimethyl butadiene with isoprene and butadiene, this was successful as the new polymer had similar properties to the natural rubber. He then replaced dimethyl butadiene with styrene and copolymerised with butadiene, the polymer was named as SBR (styrene-butadiene rubber).14
Styrene-butadiene Rubber is produced by the free-radical polymerisation of styrene mixed with butadiene. The monomers undergo free- radical polymerisation under aqueous emulsion. The monomers can also undergo polymerisation in the form of solution.31
Properties of Styrene-butadiene Rubber
The rubber has a brown -black colour. 31 The rubber has a high tensile strength meaning it does not tend to break if a low amount of tensile stress is put into it. It has a high abrasion resistance meaning the rubber will not wear out quickly. It can dissolve with organic solvents such as turpentine (which is an oiled that is attained by the distillation of resin from pine trees), ether, petrol, carbon tetrachloride, and naphtha (which is an oil attained by the distillation of coal tar). It is soluble to hydrophilic solvents such as water, acetone, and alcohol. The rubber has a moderate elastic property, this means that when the rubber is stretched out it gets bigger, but when it is released the rubber goes back into its original shape. The rubber can only be elastic between -45 C to 100 C which is better than what the given temperature range is for the Polychloroprene, this means under hot conditions the rubber would not lose its elasticity. 29
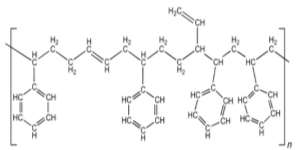
Figure 6: Styrene-butadiene rubber 32
Advantages of Styrene-butadiene Rubber
This rubber is tougher than natural rubber because it has a higher tensile strength than natural rubber meaning it does not tend to break if a low amount of tensile stress is put into it. It has a high abrasion resistance meaning the rubber will not wear out quickly. Another advantage of styrene-butadiene rubber is that it is much more cost effective than natural rubber, and the production of the rubber is much more efficient than its natural counterpart.
Disadvantages of Styrene-butadiene Rubber
It has a poor resistance to any organic compounds this is because It is soluble to organic solvents such as turpentine (which is an oiled that is attained by the distillation of resin from pine trees), ether, petrol, carbon tetrachloride, and naphtha (which is an oil attained by the distillation of coal tar). Another disadvantage of natural rubber is that it has a poor resistance to ozone meaning that when the ozone reacts with the double bonds it breaks, this causes a crack. The crack then grows steadily because of further ozone attacks so this causes the strength of the rubber to decrease massively and cause it to break. Fillers like carbon black are needed to strengthen the rubber.
Typical applications of Styrene-butadiene Rubber
- Production of car tyres
- Making mats
- Making shoe soles
Poly(Butadiene-acrylonitrile) rubbers
In 1931, scientists IG Farben developed a synthetic rubber which consisted of copolymerising acrylonitrile with butadiene, the polymer was known as NBR (Nitrile-butadiene rubber), it was the later known as Buna-N for commercial purposes. 14
Styrene-butadiene Rubber is produced by the free-radical polymerisation of styrene mixed with butadiene. The monomers undergo free- radical polymerisation under aqueous emulsion.14
Properties of Poly(Butadiene-acrylonitrile) rubbers
The rubber has a yellowish colour.33 It has a high abrasion resistance meaning the rubber will not wear out quickly. It is insoluble with organic solvents such as turpentine (which is an oiled that is attained by the distillation of resin from pine trees), ether, petrol, carbon tetrachloride, and naphtha (which is an oil attained by the distillation of coal tar). It is insoluble to hydrophilic solvents such as water, acetone, and alcohol. The rubber has a good elastic property, this means that when the rubber is stretched out it gets bigger, but when it is released the rubber goes back into its original shape. The rubber can only be elastic between -34 C to 121 C which is better than what the given temperature range is for the Polychloroprene, this means under hot conditions the rubber would not lose its elasticity. However, at low temperature, the rubber loses its elasticity.29
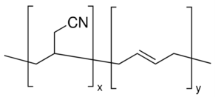
Figure 7: Poly(Butadiene-acrylonitrile)34
Advantages of Poly(Butadiene-acrylonitrile) rubbers
It has a very high resistance to hot conditions and other weather conditions; this means that it does not melt or break easily. Another advantage of Poly(Butadiene-acrylonitrile) rubber is that they have a better resistance to ozone and UV this means that less cracks forms than in its natural counterpart. This means that it is less likely to break than the natural rubber. It has a high abrasion resistance meaning the rubber will not wear out quickly. Another advantage of Poly(Butadiene-acrylonitrile) rubber is that it has a high resistance in oil this is because It is insoluble with organic solvents such as turpentine (which is an oiled that is attained by the distillation of resin from pine trees), ether, petrol, carbon tetrachloride, and naphtha (which is an oil attained by the distillation of coal tar). 29
Disadvantages of Poly(Butadiene-acrylonitrile) rubbers
At low temperature, the rubber loses its elasticity. Another disadvantage of Poly(Butadiene-acrylonitrile) rubbers, is that they have poor resistance to carbonyl compounds such as ketones and esters, and, also has a poor resistance to strong oxidising agents. 29
Typical applications of Poly(Butadiene-acrylonitrile) rubbers
- Making nitrile gloves
- Can be used for O-rings
- Can be used to make hoses and tubing
Ethylene propylene Terpolymer Rubber
In the 1960s, EPDM (which is known as Ethylene propylene Terpolymer Rubber) was produced. The rubber is made by copolymerising by a diene derivative, ethylene and propylene. The rubber has many advantageous properties over the synthetic rubbers, one of them is that they have good electrical insulating properties. EPDM rubber are now found in our everyday life such as hose, solar panels, electrical insulation etc. 16
Ethylene propylene Terpolymer Rubber is produced by the copolymerisation of ethylene, propylene and a diene derivative. The monomers under copolymerisation in the form of solution.
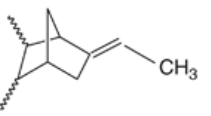
Figure 8: EPDM 35
Properties of Ethylene propylene Terpolymer Rubber
The rubber has a yellowish colour. It has a high abrasion resistance meaning the rubber will not wear out quickly. It is insoluble with organic solvents such as turpentine (which is an oiled that is attained by the distillation of resin from pine trees), ether, petrol, carbon tetrachloride, and naphtha (which is an oil attained by the distillation of coal tar). It is insoluble to hydrophilic solvents such as water, acetone, and alcohol. The rubber has a good elastic property, this means that when the rubber is stretched out it gets bigger, but when it is released the rubber goes back into its original shape. The rubber can only be elastic between -40 C to 149 C which is better than what the given temperature range is for the Poly(Butadiene-acrylonitrile) rubber, this means under hot conditions the rubber would not lose its elasticity. 29
Advantages of Ethylene propylene Terpolymer Rubber
It has a very high resistance to hot conditions and other weather conditions; this means that it does not melt or break easily so does not age very easily. Another advantage of Ethylene propylene Terpolymer rubber is that they have a better resistance to ozone and UV this means that less cracks forms than in its natural counterpart. This means that it is less likely to break than the natural rubber. It has a high abrasion resistance meaning the rubber will not wear out quickly. The rubber is very cost effective, so it is cheaper to make than the other rubber compounds. 29
Disadvantages of Ethylene propylene Terpolymer Rubber
It has a poor resistance to oils, this is because It is insoluble with organic solvents such as turpentine (which is an oiled that is attained by the distillation of resin from pine trees), ether, petrol, carbon tetrachloride, and naphtha (which is an oil attained by the distillation of coal tar).29
Typical applications of Ethylene propylene Terpolymer Rubber
- Used for door and window sealing
- Can be used for O-rings
- Can be used to make hoses and tubing
Future of rubber and conclusion
A new set of synthetic rubbers are being developed by many scientists. For example, recently, nanocomposites rubber has been discovered where scientists are using nanocomposites as reinforcing agents or fillers. Recently in 2016, a Chinese scientist named Xin Liu and his team researched on using graphene as reinforcing agent or filler and found out that the rubber has a unique and outstanding mechanical, electrical and thermal properties.36 In conclusion, it is believed that rubber plays an important part of our society, it is very important, and everyday objects need it. Leading scientists are trying to produce rubbers which are recyclable 37, this is a step in the right direction because the demand for natural rubbers and synthetic rubbers is much higher than the supply of natural rubbers and synthetic rubbers.
References
- Kong.N., 2015, A Brief history of Rubber, A Bar in a suitcase, https://oss.adm.ntu.edu.sg/nina002/2015/10/27/a-brief-history-of-rubber/, accessed on 10/12/2016
- Morawetz.H., 2000, History of Rubber Research, Rubber Chemistry and Technology, Vol. 73, No. 3, pp. 405-426.
- Rubber tree, 2012, Rainforest alliance, www.rainforest-alliance.org/species/rubber-tree/, accessed on 14/12/2016
- A little bit of history-Rubbers, 2013, Primary and early years Magazines, National centre for Excellence in the teaching of mathematics, Issue 57, pp 12
- Smeaton.W., Early attempts to melt platinum, Platinum Metals Rev, Vol 28, No 1, pp 25-30
- Knight.E., 1884, Knight’s American mechanical dictionary, J.B. Ford and company, vol 3, pp 454
- Woodford.C., 2016, Rubber, Explain that stuff, www.explainthatstuff.com/rubber.html , accessed on 14/12/2016
- Brief History of Rubber, 2010, www.iisrp.com/WebPolymers/00Rubber_Intro.pdf , accessed on 14/12/2016
- Carson, Pirie, Scott, 1893, Carson catalogue, The Verge, http://www.theverge.com/2016/12/29/14111388/google-doodle-charles-macintosh-rubber-waterproof-raincoat, accessed on 16/12/2016
- Jackson. J., 2008, The Thief at the End of the World: Rubber, Power, and the Seeds of Empire, Penguin, pp 10-432
- Trumbull. H., 1933, Accomplishments of the Medalist, Ind. Eng. Chem., Vol 25, No. 2, pp 230-232
- Sharma. B., 1991, Industrial Chemistry, Goel, pp 1127
- Smith. J, 1985, The ten-year invention: Neoprene and Du Pont Research, Society for the history of technology, Vol. 26, No. 1, pp 34-55
- Painter. P., Coleman. M., 2008, Kinetics of Chain Growth copolymerization- Buna Rubber, Essentials of Polymer Science and Engineering, pp 143
- Morris. P., 2005, Polymer pioneers, The Chemical Sciences in society, pp 54
- Ethylene-Propylene Rubbers & Elastomers, 2010, http://www.iisrp.com/webpolymers/10epdmsep11.pdf , accessed on 18/12/2016
- Koyama. T., Ohya. N., 2001, Biosynthesis of Natural Rubber and Other Natural Polyisoprenoids, Vol. 4, pp 73-81
- Natural Rubber, www.tut.fi/ms/muo/vert/5_rubber_chemistry/natural_rubber.htm, accessed on 18/12/2016
- Natural Rubber, TutorVista, http://www.tutorvista.com/content/chemistry/chemistry-ii/carbon-compounds/natural-rubber.php#properties-of-natural-rubber, accessed on 2/1/2017
- Rubber Guide to Properties, DuPont, http://www.derbygaskets.com/materials/guide%20to%20properties.pdf , accessed on 2/1/2017
- Rubber and Vulcanisation, 2006, Open Learn, http://www.open.edu/openlearn/science-maths-technology/science/chemistry/rubber-and-vulcanisation , accessed on 2/1/2017
- Rubber, 2015, New World Encyclopaedia, www.newworldencyclopedia.org/entry/Rubber , accessed on 2/1/2017
- Difference between Natural Rubber and Vulcanized Rubber, 2015, Transtutors, www.transtutors.com/chemistry-homework-help/polymers/natural-rubber-and-vulacanized-rubber.aspx , accessed on 2/11/2016
- Aclnibnib, 2016, Vulcanization, Piktochart, magic.piktochart.com/output/1377476-flow-2 , accessed on 2/1/2016
- Medical applications, 2013, Cariflex, http://www.kraton.com/products/cariflex/CariflexMedical%20Insert.pdf , accessed on 2/11/2017
- Ambasta. B., 2006, Chemistry for engineers, Laxmi, pp 225
- Introduction to rubber, 2014, Metflex, www.metflex.co.uk/wp-content/uploads/2014/10/Introduction-to-Rubber-Final.pdf , accessed on 2/1/2017
- Agarwai. O., 2009, Reactions and reagents, Goel, 46th edition, pp 399
- Material reference, 2012, J. J. Short Associates, www.jjshort.com/Rubber-Properties.php , accessed on 3/1/2017
- Polychloroprene, 2017, Sigma-Aldrich, www.sigmaaldrich.com/catalog/product/aldrich/205400 , accessed on 3/1/2017
- Emulsion Styrene-Butadiene Rubber with Oil, Sibur International, sibur-int.com/product/rubber/catalog/item251.php, accessed on 4/11/2017
- Feky. S, Tharaa. K., 2013, Styrene Butadiene Rubber, Slideshare, pp 2-9
- Misra. G., 1993, Elastomeric Materials, Introductory Polymer Chemistry, pp 137
- Poly(Acrylonitrile-butadiene), 2017, Sigma-Aldrich, www.sigmaaldrich.com/catalog/product/aldrich/180912 , accessed on 3/1/2017
- Rooj. S., Das. A., Heinrich. G., 2010, Preintercalation of an organic accelerator into nanogallaries and preparation of ethylene propylene diene terpolymer rubber clay nanocomposites, Polymer Journal, Vol. 43, pp 285-292
- Liu. X., et al, 2016, Research Progress of Graphene-Based Rubber Nanocomposites, Polymer Composites, pp 1-17
- Gailmeeblog, 2016, Synthetic Rubber: Overview and Future steps, gailmeeblog.wordpress.com/2016/04/28/synthetic-rubber-overview-future-steps/