Mesoporous Silica Nanoparticle (MNS) Properties
INTRODUCTION
1.1 Background Study
Large amount of solid remnant from agriculture industries waste can create disposal problem to environment such as generation of odour and can attract pests that can endangered human health. Due to this problem the application of this waste is widely explored to control the disposal problem. According to Norsuraya, Fazlena and Norshasyimi (2016), primary fuel source and additive in construction industries utilise the application of solid residue. The example agricultural waste is corn cob, rice husk, sugarcane leaf and bagasse. Studies have been conducted to enhance the use of this solid residue into profitable product. One of the important element present in the waste is silica that has wide application.
According to Norsuraya, Fazlena and Norshasyimi (2016), among the agriculture residue sugarcane bagasse ash (SCBA) consist the highest of the silica content with the value of 96.93%. This studies comply with the studies conducted by Rahman et al. (2015), that stated the amount of silica content in SCBA is more than 50%. Bagasse ash is the product of combustion of bagasse that are commonly used as a source energy to operate plant. Bagasse is one waste product in sugar industry that incurs additional disposal cost. Bagasse is cellular fiber remaining after extraction of the sugar-bearing juice from sugarcane. It consist of lignin (20-30 %), cellulose (40-45 %) and hemicelluloses (30-35 %) (Peng et al., 2009). The silica content varies depending on the environment, soil nature and the process involves in harvesting it.
In Malaysia the application of Sugarcane Bagasse (SCB) is still not widely explored but studies already conducted to produce silica gel as adsorbent, additive for concrete, cosmetic and others because of its characteristic. SCB is more related to by-product in sugarcane mills industry. After the juice containing sucrose called as table sugar extracted from the sugarcane by pressing the sugarcane. The residue is the SCB which contains high fibrous residue. The largest sugarcane plantation in Malaysia is at the northern region of Malaysia which can produce more than 70 000 tonne of sugarcane. The sugarcane bagasse waste from the extraction process contribute to huge disposal waste problem.
SCBA can be the most valuable stock for production of mesoporous silica that are useful application such as adsorption and catalyst (Rahman et al., 2015). For that purposes, the size and surface area of the mesoporous silica are importance. Mesoporous silica is a silica that has pore with diameter range of 2 – 50 nm depending on the process of synthesis of the mesoporous silica. The large surface area of the mesoporous silica functions as the active site that useful for the application such adsorption.
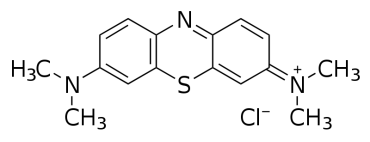
Dye is a natural or synthetic materials that can add a specific colour onto the material that are applied to. Every year more than 10 000 types of dye are produced around the world and are used in different types of industries (Malakootian et al., 2015). The application of dye used widely from food industry to textile industry with the biggest consumer of dye is textile industry. This industry is known to be the one of the main contributor of industrial wastewater pollution and it is the most polluting of all the industrial sector. 10-50% of the dye will end up in the effluent (Axelsson et al., 2006). Dye are significantly toxic and mutagenic that poses hazard to aquatic life and living organism. One of example of dye is methylene blue (MB) that are an important aromatic compound with chemical formula of C16H18ClN3S. Large amount of this dye are released in the water sources and according to Malakootian et al. (2015) it is more importance removing dye from textile wastewater than treating other colourless organic and inorganic because only a small amount of dye can affect the water quality and colour. Various treatment method introduce to removes dye from textile wastewater such as physical, chemical and biological but it is not effective due to complex chemical structure that lead to resistant to this type of treatment other than the treatment cost. Thus, it is important to find other alternative process of removal of dye.
Currently, the most known method to treat textile wastewater is by using adsorption process method because of it has more advantages compared to traditional method especially in environmental aspects and its ease of operation, cost effectiveness, biodegradability as well as greater efficiency. In order to carry out the adsorption, the main important thing is the adsorbent. According to Malakootian et al. (2015) the most typical adsorbent used for adsorption treatment for the removal of dyes from coloured water or wastewater, but due to its high production, regeneration and reactivation procedures cost research has been concentrated on alternative adsorbents with high adsorptive capacity and low cost such as mesoporous silica nanoparticle (MSN). These adsorbents have high efficiency due to their high surface area, high mechanical properties and good resistivity to thermal degradation, and they exist in several structures and amphoteric properties.
1.2 Problem Statement
The wastewater from textile contain high concentration of dye. So it required for the effluent from the industry to undergo treatment. Wastewater resulting from these industries is generally characterize by high COD, pH, dissolved solid temperature and dye and surfactant content. Dye also can be toxic to environment because of its chemical properties.
In order to treat effluent from textile industry that contain high percentage of dye, various method introduced and used. Examples of the methods are physical, chemical, biological, membrane separation, radiation, electrochemical, advanced oxidation, photolysis, electrochemical, sonolysis processes, etc. However, these methods are relatively ineffective because most dyes as azo reactive dyes are highly water soluble, have complex structures, and are stable to light, chemical and biological degradation, etc. Furthermore, these methods have certain disadvantages such as high capital and operational costs, secondary sludge disposal problems and the release of large volumes of toxic by-products (Malakootian et al., 2015).
Among all these methods, adsorption is the best method because of its advantages and the best adsorbents is by using nanotechnology method such as Mesoporous Silica Nanoparticle. It is because it has high adsorptive capacity and low cost.
1.3 Objectives of Study
The objectives of the study can be outlined as follows:
- To prepare Mesoporous Silica Nanoparticle (MSN) from bagasse ash
- To characterize the physicochemical properties of MSN.
- To study the potential of MSN as an environmental adsorbent in wastewater treatment
1.4 Research Scope
The research is to investigate the effluent of low cost adsorbent materials which is Mesoporous Silica Nanoparticle (MSN) from bagasse ash as the adsorbent to remove the Methylene Blue. The MSN are prepared from Sol-Gel Method and then Hydrothermal Synthesis by using formaldehyde, water and without formaldehyde and water.
The MSN will be characterize and will be subjected to:
- Fourier Transform Infrared Spectroscopy (FTIR) to identify the major functional chemical groups present in the silica
- Transmission Electron Microscopy (TEM) to determine the average particle size and the morphology of the materials.
- Brunauer-Emmett-Teller (BET) to evaluate the specific surface area.
- Scanning Electron Microscope (SEM) to evaluate the image of the MSN
The performance of MSN adsorbent in removing methylene blue from aqueous solution will be carried out in various parameter which are:
- Effect of pH the aqueous methylene blue in pH 3, 5, 7, 9, 11
- Effect of MSN dosage (0.1 g, 0.2 g, 0.3 g, 0.4 g and 0.5 g)
- Effect of initial concentration of aqueous methylene blue (10 ppm, 20 ppm, 30 ppm, 40 ppm and 50 ppm)
- Effect of temperature of the aqueous methylene blue (30 °C, 50 °C and 70 °C)
The treated aqueous methylene blue dye will be characterized and subjected to UV-Visible Spectrometer (UV-Vis) to evaluate the percentage of methylene blue dye remove from the aqueous solution from adsorbent by MSN.
LITERATURE REVIEW
2.1 Introduction
One of the major problem in environment is water contamination. The bad effect of water contamination will affect everything in this world such as human, animals and plants. It is because water is the main elements to support all living things and earth. Nearly all of water contamination is caused by human activities. Apart from human activities, water contamination is also caused by natural waste. From long time ago, the water contamination is studied by researcher. There are many technique and method to treat and remove contaminants in the waste water. It is for preserving both human and the environment in this world.
Among all contaminant in water, dye is one of the contaminant that exist in the water. The waster that contain dye is mainly from industry that related to textile industry, plastic and paper. It contain up to 10% of used dye. It is proven that dye is dangerous to all living things due to its harmful behaviour of dye molecules and their metabolites is significant for the development of strategies to diminish their desperate damaging impacts (Hebbar, Isloor, Zulhairun, Sohaimi Abdullah, & Ismail, 2017). In addition to having harmful adverse effect, the presence of colour in water is visually unpleasant and can destroy the entire ecosystem.
One of the most effective method to overcome the problem of effluent water containing dye is by using adsorption technique. It has been proven to be very effective by many researchers. This is because adsorption method offers more advantages compared to other method especially in environmental aspects and its ease of operation, cost effectiveness, biodegradability as well as greater efficiency.
2.2 Dyes
Dyes are produced all over the world with approximately 10 000 different type of dyes produced per year and it has been used extensively in so many industries. Most common industry that utilizing dye is textile industry and estimated to utilize around 7 Ã- 105 – 1 Ã- 106 tons (Malakootian et al., 2015). Dyes are used to dye a textile products, fur products, and others. Dye can be originated mainly from vegetables and also animal sources. There also the existence of synthetic dye that replacing the natural sources. The main function of dye are to add colour to a certain materials such as textile. Dye are also generally utilized as part of industry such as rubber, paper, cosmetic etc. Among these different industry, textile industry positions first in utilization of dyes for colouring of fiber. The dye are constantly left as major waste in these industries. Because of their compound structure, dyes are impervious to fading on presentation of light, water and numerous chemicals and this manner are hard to be decolorized once discharged into the nature or aquatic environment. Basically, dyes are ionising and aromatic compound. Inside the dyes, there are chromophores present in them. Based on their structures of the dyes, it has aryl rings that has delocalised electron systems. These structures are said to be responsible for the adsorption of electromagnetic radiation that has varying wavelengths, based upon the energy of the electron clouds.
Dye can influence aquatics life, human wellbeing and environmental framework when dye wastewaters are greatly released wastewater into water sources due to complex compound in the dye. It has big complicated molecular structure and harmful properties. It in the end rolls out improvements of ecological system and other serious pollution issues. Dye wastewaters can contain harmful organic residue with the significant mixes of phenol derivatives, aniline derivatives, organic acid and benzene derivatives (Likhar & Shivramwar, 2013)
2.2.1 Classification of Dye
Dyes can be characterized into a few classes as per their utilization, for example, reactive, disperse, direct, vat, sulphur, cationic, acid and solvent dyes. The classification of dyes according their application is as shown in table 2.1.
Table 2.1: General dyes classification and its application (Hunger, 2003)
|
Class |
Principal Substrate |
Application |
Chemical Types |
|
Acid Dyes |
Nylon, Wool, Silk, Paper, Inks and Leather |
Usually from neutral to acidic dyebaths |
Azo (including premetallised), antraquinone, triphenylmethane, azine, xanthene, nitro and nitroso |
|
Cationic (Basic Dyes) |
Paper, Polyacrylonitrile, Modified Nylon, Polyester and Inks Applied |
Applied from acidic dyebaths |
cyanine, hemicyanine, diazahemicyanine, diphenylmethane, triarylmethane, azo, azine, xanthene, acridine, oxanine and anthraquinone cotton, |
|
Direct Dyes |
Cotton, Rayon, Paper, Leather and Nylon |
Applied from neutral or slightly alkaline baths containing additional electrolyte |
Azo, phthalocyanine, stilbene and oxanine |
|
Dispersed Dyes |
Polyester, Polyamide, Acetate, Acrylic and Plastics |
Fine aqueous dispersions often applied by high temperature/ pressure or lower temperature carrier methods; dye may be padded on cloth and baked on or thermofixed |
Azo, anthraquinone, styryl, nitro and benzodifuranone |
|
Reactive Dyes |
Cotton, Wool, Silk and Nylon |
Reactive site on dye reacts with functional group on fiber to bind dye covalently under influence of heat and pH (alkaline) Solvent |
Azo, anthraquinone, phthalocyanine, formazan, oxanine |
|
Solvent Dyes |
Plastics, Gasoline, Varnishes Lacquers, Stains, Inks, Fats, Oils and Waxes |
Dissolution in the substrate |
Azo, triphenylmethane, anthraquinone and phthalocyanine cotton |
|
Sulphur Dyes |
Cotton and Rayon |
Aromatic substrate vatted with sodium sulphide and reoxidised to insoluble sulphur- containing products on fiber |
Indeterminate structure |
|
Vat Dyes |
Cotton, Rayon and Wool |
Water-insoluble dyes Solubilised by reducing with Sodium hydrogensulphide, then exhausted on fiber and Reoxidised |
Anthraquinone (including polycyclic quinines) and indigoids |
Synthetic dye are being used extensively used in different dyeing industry with textile is leading industry that utilize it with 56% of world dye production annually. Its effluent contain critical level of organic contaminants, which are toxic as it will create odour, bad taste, unsightly colour, foaming, etc. These substances are often resistant to degradation by biological methods and are not removed effectively by conventional physico-chemical treatment methods. Removal of these dyes from effluents in an economic fashion remains a major problem for textile industries.
2.2.2 Methylene Blue
Methylene Blue (MB) or also known as Methylthioninium Chloride was first synthesized at 1876 by a German Chemist, Heinrich Caro. Paul Guttman and Paul Ehrlich used MB in the treatment of Malaria dieses in 1891. During that time also, the function of MB as a dye were discovered and were used in First World War as a biological weapon and partially staining the soldiers.
MB has many uses in different field, For instance, chemists use it to detect oxidizing agents and biologists use it to stain tissue samples and detect nucleic acids. In medicine, it is used as a treatment for various illnesses and disorders, including methemoglobinemia, schizophrenia, kidney stones, and herpes infections. In aquaculture, it is used to prevent freshwater fish eggs from being infected by bacteria and fungi (“Methylene blue – New World Encyclopedia,” 2014)
In term of dye application only, MB a basic blue dye used for dyeing silk, leather, plastics, paper, and cotton mordant with tannin as well as for the production of ink and copying paper in the office supplies industry. The release of this dye to earth is troubling for both toxicological and aesthetical reasons as dye hinder light infiltration, harm the nature of the accepting streams and are toxic to food chain organisms. The dye has a synthetic origin and complex aromatic molecular structures, it is an inactive and hard to biodegrade when released into waste streams. This perspective has dependably been neglected in their discharge. The removal of synthetic dye is of incredible worry since a few dyes and their degradation products might be cancer-causing agents and poisonous and, thus, their treatment cannot rely on upon biodegradation alone.
Table 2.2: Properties of Methylene Blue (MB)
|
METHYLENE BLUE |
|
|
|
|
|
|
|
|
IUPAC name |
7-(dimethylamino)phenothiazin-3-ylidene]-dimethylazanium;chloride |
|
Properties |
|
|
Molecular formula |
C16H18ClN3S |
|
Molar mass |
319.86 g/mol |
|
Density |
43 600 mg/L at 25 °C |
|
Melting point |
100 °C |
|
Boiling Point |
Decomposes |
|
Odour |
Odourless |
|
Solubility in water |
Soluble in ethanol, chloroform; slightly soluble in pyridine; insoluble in ethyl ether |
Adapted from https://pubchem.ncbi.nlm.nih.gov/compound/methylene_blue#section=WIPO-IPC
2.2.3 Technologies for Dye Removal
Dye are used widely in all sector to colour their product. In order to colour the product large amount of dye is used. Apart to colour their product the dye also been used as paper, and plastic. This will result in large amount of effluent containing dye as contaminant into the nature. According to study conducted by Axelsson et al. (2006), 10 – 50 % of the dye used in industry will go to effluent because of the dye molecule might react with hydroxyl ions in the solution giving rise to even more water-soluble hydrolysed molecules. Because of the good solubility of dye in water it will endangered the nature. It is also reported by Sapawe et al. (2012) that 15 % of the total world production of dyes is released in textile industry. This proves that from out of 7Ã-105 tons of effluent produce large number of dye is discharges as wastewater.
Without further treatment to the effluent containing dye contaminant, it can cause extreme problems if not treated legitimately because of dyes are harmful, toxic, mutagenic, carcinogenic to human life as well to another living organism (Sapawe et al., 2012). To treat the dye so many method had been introduced and the best method reported by Malakootian et al. (2015), is by using adsorption method and supported by Marrakchi, Ahmed, Khanday, Asif, & Hameed (2017) due to some advantages. From all types of treatment, it can be classified into three categories which are divided for the technologies which are physical, chemical and biological. However, it is hard to treat the dye because of their synthetic origin and mainly complex aromatic structure. All of these technologies possess pros and cons.
2.2.3.1 Physical and Chemical Treatment
There are numerous method falls under physical and chemical treatment such as anion exchange resins, cogulations, flotation, electroflotation, electrochemical destruction, irradiation, Ozonation,adsorption, and the use of activated carbon. Physical and chemical treatment is far more effective than biological treatment in decolourizing dye but it will use more energy, chemicals, and biological process hence increasing the capital cost for the treatment (Miao, 1992). Apart from that, it will lead to secondary sludge disposal problems and the release of large volumes of toxic by-products (Malakootian et al., 2015).
2.2.3.2 Biological Treatment
Biological have three stages or phases. It is because before the effluent arriving to the biological phase, it will go through some physical and chemical treatment. For comparison to the physical and chemical method, physical and chemical treatment will treated physically or chemically without going through another phases. It is reported by Malakootian et al. (2015) that biological treatment is in effective in decolorizing water because most dyes as azo reactive dyes are highly water soluble, have complex structures, and are stable to light, chemical and biological degradation. The example of biological treatment are fungal biodegradation, bacteria biodegradation, yeast biodegradation, and microbial biosorption, Biological treatment has lower capital cost compared to physical treatment and chemical treatment (Miao, 1992).
2.3 Decolorizing of Dyes by Adsorption Process
Absorption has been proved as the best method for treatment wastewater containing dye. It offers noteworthy advantages over customary treatment techniques particularly from the environmental perspective and its simplicity of operation and also more prominent efficiency. Some adsorbents, which are utilized for the expulsion of dye from aqueous solutions with differing achievement include activated carbon, magnesium oxide grafted chitosan, modified bentonite, TiO2 powder, TiO2 nanotube and others. Among all these materials, activated carbon is a standout amongst the most much of the time adsorbents utilized for the removal of dye from coloured waters and wastewaters, yet because of its high generation, recovery and reactivation procedure cost, research has been concentrated on alternative adsorbents with high adsorptive capacity and low cost.
Therefore, recently there has been a lot of attention toward using nanotechnology methods. Nowadays using nanomethods, especially by using Mesoporous Silica Nanoparticle, The large surface area allows for binding at a great number of active sites distributed within the framework of the porous materials. The large pores can overcome the pore-diffusion limitation and provide high-speed pathways for gas molecules (Rahman et al., 2015). Apart from that, it is reported that, Mesoporous Silica Nanoparticle which is synthesized from natural sources claimed to safe in handling, cheap and can be generated from cheap resources (Norsuraya et al., 2016) which is bagasse ash in this case. Due to this advantages, the usage and studies regarding Mesoporous Silica Nanoparticle increase exponentially as adsorbent.
METHODOLOGY
3.1 Introduction
The aim of this study is to synthesis Mesoporous silica from bagasse ash by using green route and to study the performance of the Mesoporous silica in wastewater treatment by utilizing aqueous methylene blue as the wastewater. The mesoporous silica is prepared by carrying out combustion of bagasse to produce bagasse ash and then using Sol-Gel method to make gel from the bagasse ash. The product of Sol-Gel method will undergo hydrothermal synthesis by using formaldehyde, water and without formaldehyde and water to synthesis Mesoporous Silica Nanoparticle (MSN). The MSN produced will be characterize by using Fourier Transform Infrared Spectroscopy (FTIR), Transmission Electron Microscopy (TEM), Brunauer-Emmett-Teller (BET), Scanning Electron Microscope (SEM). The performance study for wastewater treatment to treat aqueous methylene blue by using MSN will be test under four parameter which is the effect of pH, effect of MSN dosage, effect of initial concentration of the dye, and effect of temperature.
The research methodology is summarized in the research flow chart in Figure 3.1 below.
3.2 Material and Methods
In this study there are six stages of preparations and experiment. Which are the preparation of bagasse ash, preparation of silica gel from bagasse ash, production of Mesoporous Silica Nanoparticle (MSN), characterization of the MSN, Performance study of MSN and characterization of treated wastewater.
3.2.1 Preparation of Bagasse ash
The material, apparatus and glassware needed for this stage as shown in table 3.1
Table 3.1: List of Materials, Glassware and Apparatus
|
Materials |
Apparatus |
|
Sugarcane Bagasse |
Oven |
|
Furnace |
Sugarcane Bagasse can be collected from sugarcane juice hawker at Tampin, Negeri Sembilan. First step need to be taken is to cut the bagasse in small pieces and boil the bagasse to remove the remaining sugar in the bagasse, after that the bagasse need to be wash and rinse by using distilled water to remove impurities. After that, the bagasse will be subjected to oven drying process at 70°C – 80 °C for 24 hours. To obtain ash from the bagasse, combustion are to be carried out in furnace at 800 °C for 3 hours at heating rate of 10 °C/min. The ash need to be collected and labelled as Sugarcane Bagasse Ash (SBA).
3.2.2 Preparation of Silica Gel from Bagasse Ash (Sol-Gel Method)
The material, apparatus and glassware needed for this stage as shown in table 3.2
Table 3.2: List of Materials, Glassware and Apparatus for Sol-Gel Method
|
Materials |
Apparatus |
|
SBA |
Erlenmeyer Flask |
|
1M Lye Solution (NaOH) (1 litre) |
Stirrer |
|
Distilled Water |
Beaker |
|
1M Sulphuric Acid (H2SO4) |
Pipette (25 ml capacity) |
|
pH meter |
Boil 30g of Bagasse Ash and 1 L of 1M Lye Solution (NaOH) in Erlenmeyer flask for 1 hour with a constant stirring to dissolves the silica and produce a sodium silicate solution. Filter the solution through whatman No. 41 ashless filter paper and wash the residue with boiled distilled water. Let the filtrate to cool to room temperature. Reduce the pH of the solution to pH 7 by using 1M Sulphuric Acid (H2SO4) by using titration method and constant stirring. When gel form from the solution from the solution, age it for approximately 18 hours. After the ageing, gently broke the gel and centrifuge at 2500 rpm for 10 minutes. Discard the supernatant and transfer the gel into a beaker and dry for 11-13 hours at 80°C to produce xerogels. Wash with deionized water to remove minerals and impurities from the silica
3.2.3 Production of Mesoporous Silica Nanoparticle (Hydrothermal Synthesis)
The material, apparatus and glassware needed for this stage as shown in table 3.3
Table 3.3: List of Materials, Glassware and Apparatus for Hydrothermal Synthesis
|
Materials |
Apparatus |
|
Cetyltrimethylammonium Bromide (CTAB) |
Conical Flask |
|
Ammonium Hydroxide (NH4OH) |
Stirrer |
|
Distilled Water |
Beaker |
|
Microwave |
Mix 0.64 g of cetyltrimethylammonium bromide (CTAB) and 30 ml formaldehyde solution (37 wt %) in 100 ml closed conical flask and stir at 27°C for 5 minutes. Quickly add 2.8 ml NH4OH and stir for 30 minutes. Add 2.8 ml of silica prepared in Sol-Gel method and stir vigorously for 24 hours. Transfer the mixture to 100 ml beaker and place it in microwave at 800 Watt for 30 minutes. Filter the product and wash with deionize water and dried it overnight at 60°C. Calcinate the dried product in air at 540°C for 3 hours to remove surfactants in order to obtain MSNs. Repeat all step by replacing formaldehyde with water and without formaldehyde. The product form is Mesoporous Silica Nanoparticle (MSN).
3.2.4 Characterization of MSN
In order to characterize the MSN, analytical instrument needed as shown in table 3.4
Table 3.4: List of analytical instrument for MSN characterization
|
Instrument |
|
Fourier Transform Infrared Spectroscopy (FTIR) |
|
Transmission Electron Microscopy (TEM) |
|
Brunauer-Emmett-Teller (BET) |
|
Scanning Electron Microscope (SEM) |
Fourier-transform infiltered (FT-IR) spectra were collected on a Nicolet Fourier spectrophotometer in the wave length range of 400-4000 cm-1 using KBr pellets. FTIR are used to identify the major chemical groups present in the silica.
Transmission electron microscopy (TEM) images were recorded on a Tecnai G2 20 S-twin instrument (FEI Company) with an acceleration voltage of 200 kV. The samples for TEM analysis were prepared by dipping carbon-coated copper grids into ethanol solutions of MSNs and drying at ambient condition. TEM images are to determine the average particle size and the morphology of the materials
AJSM-7500F FESEM instrument (JEOL, Peabody,MA, USA) was used for SEM analysis to evaluate the image of the MSN.
The specific surface areas were evaluated using the Brunauer-Emmett-Teller (BET) method and the pore size distributions were calculated using the Barrett-Joyner-Halenda (BJH) method
3.2.5 Preparation of Dye Solution
The material, apparatus and glassware needed for this stage as shown in table 3.5.
Table 3.5: List of Materials, Glassware and Apparatus for Preparation of Dye Solution
|
Materials |
Apparatus |
|
Methylene Blue |
Beaker (1.5 L) |
|
Distilled Water |
Stirrer |
Prepare 10 ppm, 20 ppm, 30 ppm, 40 ppm and 50 ppm dye solution by mixing 0.01, 0.02, 0.03, 0.04 and 0.05 g dye (Methylene Blue) respectively with 1 L of distilled water in 1.5 L beaker. Batch sorption are to be conducted in 100 ml beaker with 0.2 g of MSNs and 50 ml of dye solution
3.2.6 Performance Study of MSN towards Wastewater Treatment
The performance of the MSN towards treating wastewater is tested in four parameter which is effect of pH, effect of MSN dosage, effect of initial concentration of dye solution and the effect of the temperature of solution.
3.2.6.1 Effect of pH
The effect of pH solution on dye removal is 3, 5, 7, 9, 11 with initial concentration of dye solution 20 ppm and weight of MSN sorbent 0.2 g. The pH of the solution was adjusted using 0.1 M HCl or 0.1 M NaOH solutions. Suspend 0.2g of MSN in 50 ml of 20 ppm dye solution that has different pH value. The contact time is 10, 20, 30, 60, 90 and 120 minutes. Evaluate the extent of reduction by using UV-VIS spectrometer by measuring the maximum absorbance at 586 nm using Perkin-Elmer Lambda 35 UV-Visible spectrophotometer
3.2.6.2 Effect of MSN dosage
Mix 5 different weight (0.1 g, 0.2 g, 0.3 g, 0.4 g and 0.5 g) of MSN with 20 ppm dye solution. The contact time is 30, 90, 180 and 240 minutes. Evaluate the extent of reduction by using UV-VIS spectrometer by measuring the maximum absorbance at 586 nm using Perkin-Elmer Lambda 35 UV-Visible spectrophotometer.
3.2.6.3 Effect of Initial Concentration
0.2 g of MSNs mix with dye solution with the prepared different concentration. The contact time is 10, 20, 30, 50, 70 and 100 minutes. Evaluate the extent of reduction by using UV-VIS spectrometer by measuring the maximum absorbance at 586 nm using Perkin-Elmer Lambda 35 UV-Visible spectrophotometer.
3.2.6.4 Effect of Temperature
Prepare 5 sets of mixture of 0.2 g MSN with 20 ppm dye solution. Let the mixture in 5 different temperature which is 30 °C, 50 °C and 70 °C. The contact time is 30, 90, 180 and 240 minutes. Evaluate the extent of reduction by using UV-VIS spectrometer by measuring the maximum absorbance at 586 nm using Perkin-Elmer Lambda 35 UV-Visible spectrophotometer
3.2.7 Characterization of Treated Dye Solution
Concentration of dye solution after treated by MSN is measured by using the maximum absorbance at 586 nm using Perkin-Elmer Lambda 35 UV-Visible spectrophotometer. Calibration curve need to be determine by scanning series of dye solutions with different concentrations. A linear relationship between the absorbance and MV concentrations. Linear relationship between the absorbance and dye solution concentration can be obtained. The concentration of dye solution can be obtained from the calibration curve.
The percentage of absorbance can be calculated as follows;
ADS = 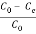 Ã- 100%
Ã- 100%
Where
C0 = Initial Concentration
Ce = Equilibrium Concentration
Â
REFERENCES
Axelsson, J., Nilsson, U., Terrazas, E., Alvarez Aliaga, T., & Welander, U. (2006). Decolorization of the textile dyes Reactive Red 2 and Reactive Blue 4 using Bjerkandera sp. Strain BOL 13 in a continuous rotating biological contactor reactor. Enzyme and Microbial Technology, 39(1), 32-37. https://doi.org/10.1016/j.enzmictec.2005.09.006
Hebbar, R. S., Isloor, A. M., Zulhairun, A. K., Sohaimi Abdullah, M., & Ismail, A. F. (2017). Efficient treatment of hazardous reactive dye effluents through antifouling polyetherimide hollow fiber membrane embedded with functionalized halloysite nanotubes. Journal of the Taiwan Institute of Chemical Engineers, 72, 244-252. https://doi.org/10.1016/j.jtice.2017.01.022
Hunger, K. (2003). Industrial dyes: chemistry, properties, applications. John Wiley and Son.
Likhar, M. C., & Shivramwar, M. V. (2013). Removal of chemical oxygen demand ( COD ) and color from dye manufacturing industry by coagulation, 3(2), 1116-1118.
Malakootian, M., Mansoorian, H. J., Hosseini, A., & Khanjani, N. (2015). Evaluating the efficacy of alumina/carbon nanotube hybrid adsorbents in removing Azo Reactive Red 198 and Blue 19 dyes from aqueous solutions. Process Safety and Environmental Protection, 96, 125-137. https://doi.org/10.1016/j.psep.2015.05.002
Marrakchi, F., Ahmed, M. J., Khanday, W. A., Asif, M., & Hameed, B. H. (2017). Mesoporous-activated carbon prepared from chitosan flakes via single-step sodium hydroxide activation for the adsorption of methylene blue. International Journal of Biological Macromolecules, 98, 233-239. https://doi.org/10.1016/j.ijbiomac.2017.01.119
Methylene blue – New World Encyclopedia. (2014). Retrieved March 15, 2017, from http://www.newworldencyclopedia.org/entry/Methylene_blue
Miao, Y. (1992). Biological remediation of dyes in textile effluent : a review on current treatment technologies. Water, 1-10.
Norsuraya, S., Fazlena, H., & Norhasyimi, R. (2016). Sugarcane Bagasse as a Renewable Source of Silica to Synthesize Santa Barbara Amorphous-15 (SBA-15). Procedia Engineering, 148, 839-846. https://doi.org/10.1016/j.proeng.2016.06.627
Peng, F., Ren, J. L., Xu, F., Bian, J., Peng, P., & Sun, R. C. (2009). Comparative study of hemicelluloses obtained by graded ethanol precipitation from sugarcane bagasse. Journal of Agricultural and Food Chemistry, 57(14), 6305-6317. https://doi.org/10.1021/jf900986b
Rahman, N. A., Widhiana, I., Juliastuti, S. R., & Setyawan, H. (2015). Synthesis of mesoporous silica with controlled pore structure from bagasse ash as a silica source. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 476, 1-7. https://doi.org/10.1016/j.colsurfa.2015.03.018
Sapawe, N., Jalil, A. A., Triwahyono, S., Adam, S. H., Jaafar, N. F., & Satar, M. A. H. (2012). Isomorphous substitution of Zr in the framework of aluminosilicate HY by an electrochemical method: Evaluation by methylene blue decolorization. Applied Catalysis B: Environmental, 125, 311-323. https://doi.org/10.1016/j.apcatb.2012.05.042