Novel Dihydroquinoline Derivatives Facile Synthesis
Facile synthesis of novel dihydroquinoline-3,3-dicarbonitriles in the presence of glacial aceticacid as catalyst under solvent-free conditions
Masoud Nasr-Esfahani* and Elham Kanaani
Department of Chemistry, Yasouj University, Yasouj, Iran
Abstract
A series of novel dihydroquinoline derivatives were synthesized using malononitrile, 2-aminobenzoic acid and benzaldehydes in the presence of a catalytic amount of acetic acid, without the use of any additional co-catalyst, under solvent-free conditions. The reaction is characterized by high efficiency, easy workup, simple purification of the products and availability of catalyst.
Keywords: Dihydroquinoline derivatives, Acetic acid, Malononitrile, 2-Aminobenzoic acid, solvent-free
Introduction
Heterocyclic compounds including nitrogen, have an important role in organic chemistry. Among these compounds, the quinoline derivatives have attracted great attention because of their application in biological and pharmacological fields. They act as antimalarial,[1-3] anti-psychotic,[4] antihypertensive,[5] anti-parasitic,[6] anthelmintic,[7] antitubercular,[8] antiasthmatic,9] antifungals,[10,11] anticancer,[12] anti-inflammatory,[13] anti-HIV,[14] anti-AIDS,[15] and antineoplastic.[16]A few promising compounds with quinoline ring system are shown as 1–3 compounds (Fig. 1). Furthermore, quinoline derivatives can be used in the synthesis of fungicides, biocides, alkaloids and flavoring agents,[17] as well as these compounds find use in manufacturing a wide variety of food and lake colors. They could generate a sharp green electroluminescence and have the high quantum efficiency of emission in the blue and the green region.[18] Therefore, in regard to these observations and importance of pharmaceutical and biological of these compounds, herein we study the solvent-free synthesis of novel dihydroquinoline derivatives in presence of glacial acetic acid as catalyst.
In the context of green chemistry, the development of clean technologies is very important in organic and medicinal chemistry. The use of available and nontoxic catalysts and replacing solution reactions with solvent-free ones are some cases that can help reduction and elimination of harmful effects of chemical reactions.[19]
The volatile nature and toxicity of many organic solvents that are widely used for organic reactions have propounded a serious threat to the environment. Therefore, in recent years, the design of solid-state reaction has received much attention from the eco-friendly synthesis viewpoint. Solvent-free techniques represent several significant synthetic benefits including savings in money, time and products, and simplicity of the experimental procedure and work-up technique.
In recent times application of nontoxic catalysts such as glacial acetic acid in chemical reactions has been an area of interest. Acetic acid is an excellent polar protic solvent and can act as a mild and efficient catalyst for the promotion of the organic reactions. Other factors that stimulate the use of acetic acid include the price of catalyst and simplicity of the work-up procedure.
In this research, we report the synthesis of 4-oxo-2-aryl-1,2-dihydroquinoline-3,3(4H)-dicarbonitriles, that involves two steps, in presence of glacial acetic acid under solvent-free conditions. AcOH is an efficient, inexpensive and available acid and in recent decades has been recognizing as a mild catalyst in organic synthesis.[20]
Results and Discussion
In continuation of our studies in the development of the synthetic methodologiesfor the preparing of fine chemicals and heterocyclic compounds of biological importance,[21-25] herein, we were interested in reporting the synthesis of novel dihydroquinoline derivatives in the presence of the glacial acetic acid as a mild and efficient catalyst. This synthesis involves two steps: firstly, 2-(2-aminobenzoyl) malononitrile intermediate (6) was synthesized via the glacial acetic acid-catalyzed reaction of 2-aminobenzoic acid (4) with malononitrile (5) under solvent-free condition. Subsequently, the novel dihydroquinoline derivatives (8)were prepared by addition of benzaldehyde derivatives (7) to the mixture reaction and attack on the intermediate 6 and followed by intermolecular cyclization (Scheme 1, Table 1).
The main advantage ofthis reaction that was carried out with AcOH is that the percentage of peripheral products was low and the recrystallization was also much easier.
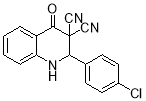
The 1H NMR spectrum of 8b showed a singlet identified as CH (δ = 4.263 ppm), and a signal at δ 7.831 ppm for NH group. The signals appearing in the 7.308-8.197 ppm are assigned for aromatic rings protons. The proton decoupled 13CNMR spectrum of 8b compound exhibited 14 distinct resonances that confirmed the proposed structure.
The infrared spectra (IR) of these compounds show NH bonds appearing at 3388-3453 cm-1. The bands found at 2210-2229 cm-1 are attributed to the CN groups. The intense bands appearing at 1695-1700 cm-1 are assigned to carbonyl groups. The peaks in the region of 1025-1350 cm-1 are assigned for Ï… (C-N) stretching vibration.
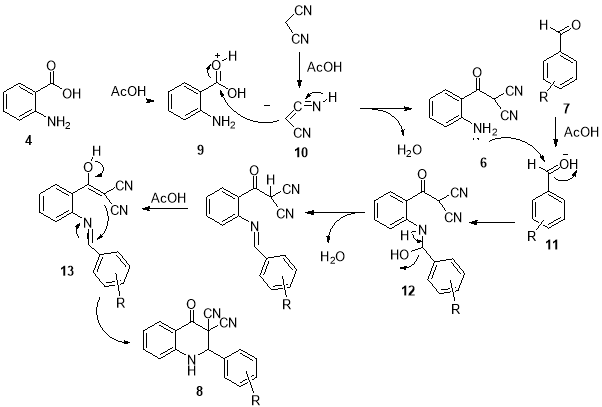
The proposed mechanism in which acetic acid has catalyzed this conversion was depicted in Scheme 3. Initially, the proton of acetic acid activates carbonyl group of 2-aminobenzoic acid (3) to achieve intermediate 9 and thus increases the electrophilicity carbonyl carbon of acid. In the following, nucleophilic addition of intermediate 10 was done by intermediate 9 and following the loss of H2O intermediate 6 was produced. In the next step, with the addition of an aromatic aldehyde to the reaction mixture, the carbonyl group of aldehyde was activated by acetic acid to give intermediate 11 thus increases the electrophilicity of carbonyl carbon of aldehyde 7 . The reaction proceeds by nucleophilic addition of the amino group of 6 to the activated aldehyde to afford intermediate 12 and following loss of H2O intermediate 13 was produced. Finally, with intermolecular cyclization of intermediate 13 the product 8 was produced (Scheme 2).
Conclusions
In summary, a novel class of dihydroquinoline derivatives 8 was obtained using 2-aminobenzoic acid, malononitrile and aromatic aldehydes in presence of AcOH as catalyst under solvent-free conditions. These novel compounds as potentially useful compounds with possible biological and pharmaceutical activities can be applied in various fields such as medicinal and agricultural areas. The most important features of this protocol are an inexpensive and available catalyst, simple purification, easy work-up, with the desired products being isolated in excellent yields.
Experimental Section
Chemicals and reagents were purchased from Merck, Fluka, and Aldrichchemical companiesand were used without further purification. IR spectra were recorded applying a FT-IR JASCO-680 spectrophotometer in KBr with absorptions in cm-1. The 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Bruker 400 MHz Ultrashield spectrometer in DMSO-d6 solution with TMS as an internal standard. Mass spectra were recorded by the Fisons Trio 1000 (70 ev). All melting points were measured on a Barnstead Electrothermal (BI 9300) apparatus in open capillary tubes and all are uncorrected. The progress of the reaction was monitored by thin layer chromatography (TLC).
General procedure for the synthesis of dihydroquinoline derivatives using AcOH
Firstly, a mixture of malononitrile 5 (1.0 mmol, 0.06 g), 2-aminobenzoic acid 4 (1.0 mmol, 0.14 g) and glacial acetic acid (o.2 ml), was heated at 80 °C under solvent-free conditions with concomitant stirring for the 6 h (reactions were monitored by TLC). Subsequently, with the formation of intermediate 6, aromatic aldehyde 7 (1.0 mmol) was added to the reaction mixture, and the mixture was stirred under reflux for the suitable time (reactions were monitored by TLC). After completion of the reaction, ethyl acetate was added and the obtained mixture filtered and then washed with water. After that, the obtained crude products were recrystallized in ethyl acetate to afford the pure product in 70-87% yields (table 1). The products were characterized by IR, 1H NMR, 13C NMR and mass spectroscopic methods.
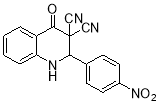
2-(4-nitrophenyl)-4-oxo-1,2-dihydroquinoline-3,3(4H)-dicarbonitrile (8a):
Brown solid, Mp: 238-240 °C;IR (KBr, cm-1): 3440, 3165, 2225, 1695, 1509, 1417, 1344, 1203, 1160, 833, 572; 1H NMR (400 MHz, DMSO-d6): 8.39 (t, 2H, J = 7.8 Hz, aromatic CH), 8.30 (d, 1H, J = 7.6 Hz, aromatic CH), 8.15 (t, 2H, J = 7.8 Hz, aromatic CH), 8.07 (s, 1H, NH), 7.91 (t, 1H, J = 8.4 Hz, aromatic CH), 7.69-7.63 (m, 2H, aromatic CH ), 4.62 (s, 1H, CH); 13C NMR (100 MHz, DMSO-d6): 203.81, 162.54, 149.23, 148.75, 138.52, 131.44, 129.52, 126.17, 124.65, 118.15, 116.19, 111.06, 60.24, 56.02; MS (m/z): 318.1[C17H10N4O3]+, 293.1 [C16H11N3O3]+, 246.1 [C16H12N3]+, 234.1 [C16H12NO]+, 184.1 [C11H8N2O]+, 277, 170, 127, 101, 89, 75.
Acknowledgements
We are grateful to the Yasouj University for supporting this work.
SUPPORTING INFORMATION
Experimental method, IR, 1H NMR, 13C NMR, Mass and MP for this article can be found via the “Supplementary Content” section of this article’s webpage.
Broom, A. D.; Shim, J. L.; Anderson, G. L. J. Org. Chem. 1976, 41, 1095.
References
- Kaur, K.; Jain, M.; Reddy, R. P.; Jain, R. Eur. J. Med. Chem. 2010, 45, 3245-3264.
- Marella, A.; Tanwar, O. P.; Saha, R.; Ali, M. R.; Srivastava, S.; Akhter, M.; Shaquiquzzaman, M.; Alam, M. M. Saudi. Pharm. J. 2013, 21, 1-12.
- Wang, X. S.; Zhang, M. M.; Jiang, H.; Yao, C. S.; TU, S. J. Tetrahedron 2007, 63, 4439-4449.
- Li, K.; Li, Y.; Zhou, D.; Fan, Y.; Guo, H.; Ma, T.; Wen, J.; Liu, D.; Zhao, L. Bioorg. Med. Chem. 2016, 24,1889-1897.
- Eswaran, S.; Adhikari, A. V.; Chowdhury, I. H.; Pal, N. K.; Thomas, K. D. Eur. J. Med. Chem. 2010, 45, 3374-3383.
- Ulahannan, R. T.; Panicker, C. Y.; Varghese, H. T.; Musiol, R.; Jampilek, J.; Alsenoy, C. V.; War, J. A.; Srivastave, S. K. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2015, 151, 184-197.
- Ortiz-Cervantes, C.; Flores-Alamo, M.; Garcia, J. J. Tetrahedron lett. 2016, 57, 766-771.
- Almansour, A. I.; Arumugam, N.; Kumar, R. S.; Menendez, J. C.; Ghabbour, H. A.; Fun, H. K.; Kumar, R. R. Tetrahedron lett. 2015, 56, 6900- 6903.
- Ghaffari Khaligh, N. Chin. J. Catal. 2014, 35, 474-480.
- Safaei-Ghomi, J.; Ghasemzadeh, M. A. J. Nanostruct. 2012, 1, 243-248.
- R. Musiol, J. Jampilek, V. Buchta, L, Silva, H, Niedbala, B. Podeszwa, A. Palka, K. Mejerz- Maniecka, B. Oleksyn, J. Polanski, ‘Antifungal properties of new series of quinoline derivatives’, Bioorg. Med. Chem. 2006, 14, 3592- 3598.
- M. M. Ghorab, F. A. Ragab, H. I. Heiba, R. K. Arafa, E. M. El-Hossary, ‘In vitro anticancer screening and radiosensitizing evaluation of some new quinolines and pyrimido[4,5-b] quinolines bearing a sulfonamide moiety’, Eur. J. Med. Chem. 2010, 45, 3677-3684.
- Ch. Yu, H. Zhang, Ch. Yao, T. Li, B. Qin, J. Lu, D. Wang, ‘One-pot three-component synthesis of benzo[f]thiopyrano[3,4-b]quinolin-11(8H)-one derivatives’, J. Heterocycl. Chem. 2014, 51, 702-705.
- J. H. Peng, R. H. Jia, N. Ma, G. Zhang, F. Y. Wu, ‘A facile and expeditious microwave-assisted synthesis of furo [3,4-b]indeno[2,1-f]quinolin-1-one derivatives via multicomponent reaction’, J. Heterocycl. Chem. 2013, 50, 899-902.
- C. Benard, F. Zouhiri, M. Normand-Bayle, M. Danet, D. Desmaele, H. Leh, J. F. Mouscadet, G. Mbemba, C. M. Thomas, S. Bonnenfant, M. Le Bret, J. d’Angelo, ‘Linker-modified quinoline derivatives targeting HIV-1 integrase: synthesis and biological activity’, Bioorg. Med. Chem. Lett. 2004, 14, 2473-2476.
- X. Xu, W. Liu, Zh. Wang, Y. Feng, Y. Yan, X. Zhang, ‘Silver-catalyzed one-step synthesis of multiply substituted quinolines’, Tetrahedron Lett. 2016, 57, 226-229.
- S. P. Shirame, S. Y. Jadhav, R. B. Bhosale, ‘Design and synthesis of 1,2,3- triazole quinoline analogues via click chemistry approach and their antimicrobial, antioxidant activites’, Asian J. Pharm. Clin. Res. 2014, 7, 163-165.
- Y. T. Tao, E. Balasubramanian, A. Danel, B, Jarosz, P. Tomasik, ‘Sharp green electroluminescence from IH-pyrazolo[3,4,b]quinoline-based light-emitting diodes’, Appl. Phys. Lett. 2000, 77, 1575-1577.
- M. Nasr-Esfahani, M. Montazerozohori, M. Taei, ‘Aluminatesulfunic acid: Novel and recyclable nanocatalyst for efficient synthesis of aminoalkyl naphthols and amidoalkyl naphthols’, C. R. Chim. 2016, 19, 986-994.
- M. El-Sayed, K. Mahmoud, A. Hilgeroth, ‘Glacial acetic acid as an efficient catalyst for simple synthesis of dindolymethans’, Curr. Chem. Lett. 2014, 3, 7-14.
- M. Nasr-Esfahani, Z. Rafiee, M. Montazerozohori, H. Kashi, ‘A highly efficient magnetic solid acid nanocatalyst for the synthesis of new bulky heterocyclic compounds’, RSC Adv. 2016, 6, 47298- 47313.
- M. Nasr-Esfahani, M. Montazerozohori, M. Aghel-Mirrzaee, H. Kashi, ‘Efficient and green catalytic synthesis of dihydropyrimidinone (thione) derivatives using cobalt nitrate in solvent-free conditions’, J. Chil. Chem. Soc. 2014, 1, 2311-2314.
- M. Nasr-Esfahani, S. J. Hosseini, M. Montazerozohori, R. Mehrabi, H. Nasrabadi, ‘Magnetic Fe3O4 nanoparticles: efficient and recoverable nanocatalyst for the synthesis of polyhydroquinolines and hantzsch 1,4-dihydropyridines under solvent-free conditions’, J. Mol. Catal. A: Chem. 2014, 382, 99-105.
- M. Nasr-Esfahani, T. Abdizadeh, ‘Nanorod vanadatesulfuric acid as a novel, recyclable and heterogeneous catalyst for the one-pot synthesis of tetrahydrobenzopyrans’, J. Nanosci. Nanotechnol. 2013, 13, 5004- 5001.
- M. Nasr-Esfahani, S. J. Hosseini, F. Mohammadi, ‘Fe3o4 nanoparticles as an efficient and magnetically recoverable catalyst for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones under solvent-free conditions ‘, Chin. J. Catal. 2011, 32, 1484-1489.
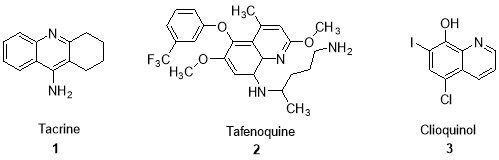
Figure 1: promising compounds with quinoline ring
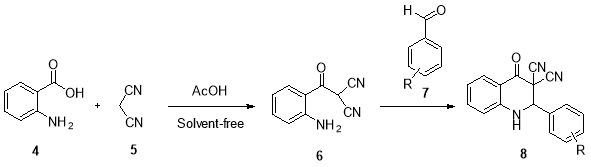
Scheme 1:Synthesis ofdihydroquinoline-3,3-dicarbonitrile derivatives
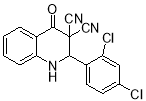
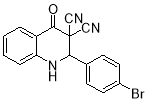
Table 1. Synthesis of 4-oxo-2-aryl-1,2-dihydroquinoline-3,3(4H)-dicarbonitriles using AcOH
|
Entry |
R |
Product |
Time 1 (h) |
Time 2 (h) |
Yield (%) a |
Mp (°C) |
|
8a |
4-NO2 |
|
6 |
5 |
87 |
238-240 |
|
8b |
4- Cl |
|
6 |
6 |
87 |
201-204 |
|
8c |
2,4- Cl2 |
|
6 |
6 |
84 |
177-179 |
|
8d |
4- Br |
|
6 |
8 |
74 |
217-225 |
|
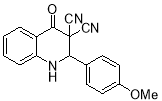
8e |
4- OMe |
|
6 |
9 |
77 |
206-208 |
|
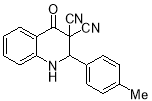
8f |
4- Me |
|
6 |
9 |
69 |
140-142 |
a Isolated yield.
Scheme 2: Proposed mechanism for the formation of dihydroquinolines 8.