Phase Transformations & Microstructural Control
Phase transformations & microstructural control
2xxx-series Aluminum Alloys
Introduction:
2000 Series Aluminum Alloys: Principal alloying element is copper with minor additions of manganese and
magnesium. This series of aluminum is the original heat treatable alloy group developed in the 1920’s. The
best known, most widely used heat treatable alloy for aircraft and aerospace is 2024. Can be spot and
friction welded but not fusion welded (a few exceptions being tank structures in the Titan Missile). Has good
formability in the annealed temper condition and some formability in the solution treated and aged condition,
but needs intelligent application in complex designs. Has excellent fatigue properties when compared to
other aluminum alloys, excellent strength to weight ratio. Good machinability. Poor resistance to corrosion
without alclad layer or secondary chem film, anodize and/or prime and paint. Can be chem film and anodized
readily. Other 2000 series alloys include 2017 seen widely in aluminum rivets, fasteners and screw machine
parts and 2014 which is used heavily in forgings. These three alloys, 2024, 2014, and 2017 can be
considered the foundations of aluminum aircraft, missiles and space vehicles during these past 75 years.
Heat Treating Aluminum Alloys: (1)
Example 2024:
Aluminum alloys are not allotropic they do not undergo a phase or structure change like steels when heating.
But if the right alloying additions are present they can be heat treated by solution heat treating and
precipitation hardening. In the early days (1930’s) solution heat treatment was referred to as ST, and many
times precipitation hardening was referred to as aging.
Solution heat treatment involves temperatures very close to the melting point of the aluminum alloy, usually
200 300 deg. below the melting point. The purpose is to provide enough thermal energy to dissolve, in a
solid solution, the alloy elements present. In the case of 2024, the major alloy element is copper, and by
taking the part to 920 deg. F, the copper present within the 2024 will dissolve or disperse uniformly
throughout the solid aluminum part. This can be difficult to comprehend how can something dissolve and still
be solid? As Einstein once said, “…everything is relative…” Without getting into the solid state physics of the
metallurgical reactions, dissolution does occur but only at this high temperature. However, if you slowly cool
down the part, the copper wants to come back out of solution. Here is where the important step of
quenching takes place. Quenching is a very rapid cool down, using water, on the order of 500 600 deg. per
second. Quenching locks in place all alloy elements that have been dissolved at the high solution heat treat
temperature. Before the alloy additions can think about changing places and moving back out of solution
wham! they are locked in place by the rapid quench cool down. The result is called a “super saturated solid
solution” an unstable condition. Quenching is critical to proper solution heat treatment.
Aging Precipitation Hardening can now happen under the right conditions. In the case of natural aging of
2024, or aging at room temperature, the dissolved copper slowly comes back out of solution over an
extended time (96 hours minimum), forming CuAl precipitants. Precipitants or precipitated particles can be
thought of as army commandos, coming from nowhere out of the sky to stand guard, strengthening the
territory. Indeed the word precipitant comes from the weather term precipitation meaning to separate and
fall from solution (clouds). Precipitated particles in heat treatable aluminum alloys strengthen the alloy by
pinning or locking up numerous microstructural features in the aluminum. Other heat treatable alloys like
6061 and 7075 undergo very similar precipitation reactions, with the actual precipitated particles differing
depending on whether zinc, magnesium, manganese, silicon or copper additions are present. The way that
metallurgists control the formation of these precipitants will determine the mechanical and corrosion
properties later.
In the case of artificial aging or precipitation hardening, the previously solution heat treated and quenched
parts are subjected to elevated temperatures (instead of room temperature) in the range of 225-375 deg. F
over extended periods of time (4-24 hours). The precipitants formed and grown here are more controlled
and substantial in nature, resulting in higher mechanical properties as compared to naturally aged
conditions.
Abstract:
Recently, Aluminum and Aluminum alloys are broadly used in several fields of industries due to their properties such as, light weight (low density), good formability, good malleability, high electrical conductivity, high thermal conductivity and high corrosion resistance. In general, pure aluminum and its alloys still have many problems during using in the engineering applications; for example: unstable mechanical properties and relatively low strength . Therefore; adding alloying elements, and heat treatment are done to modify the microstructure and improve the mechanical properties of aluminum. The alloying elements could be classified as major and minor elements, microstructure modifiers or impurities, where the minor elements in some alloys may be major elements in other alloys. This report investigates the influences of copper as alloying element on aluminum alloys and then the microstructures – mechanical properties relations in this regards.
Introduction:
Aluminum – copper alloys are gaining huge industrial usage because of their outstanding combination of mechanical, physical properties. These properties involve high specific strength specially high temperature strength, high hardness. These properties obtained through addition of copper and other alloy elements and heat treatment ability of this series. Alloying elements are chosen according to their effects and suitability (1)
Aluminium-Copper Alloys:
Due to the , aluminium – copper alloys are designated 2xxx series. the major alloying element in this series is copper (Cu).
2xxx series could include manganese (Mn), magnesium (Mg), Silicon (Si), titanium (Ti), and nickel (Ni) as minor alloying elements.
Table (1) shows the chemical compositions of some wrought aluminum-copper alloys (2xxx) (2):
Table (1): chemical compositions of Al-Cu alloys
|
Alloy |
Si% |
Cu% |
Mn% |
Mg% |
Ni% |
Ti% |
Other elements% |
|
|
|
0.4 max |
5.0 – 6.0 |
– |
– |
– |
– |
Pb=0.4 Bi=0.4 |
|
|
|
0.5 – 1.2 |
3.9 – 5.0 |
0.4 – 1.2 |
0.2-0.8 |
– |
0.15 max |
– |
|
|
|
0.2 – 0.8 |
3.5 – 4.5 |
0.4 – 1.0 |
0.4 – 0.8 |
– |
0.15 max |
– |
|
|
0.9 max |
3.5-4.5 |
– |
0.4-0.9 |
1.7-2.3 |
– |
– |
|
|
|
0. 5 max |
3.8 – 4.9 |
0.3 – 0.9 |
1.2 – 1.8 |
– |
0.15 max |
– |
|
|
2025 |
0.5-1.2 |
3.9-5.0 |
0.4-1.2 |
– |
– |
0.15 max |
– |
|
|
2124 |
0. 2 max |
3.8 – 4.9 |
0.3 – 0.9 |
1.2 – 1.8 |
– |
0.15 max |
– |
|
|
|
0. 2 max |
5.6 – 6.8 |
0.2 – 0.4 |
0.02 – 0.1 |
V=0.1 Zr=0.18 |
|||
|
2319 |
0. 2 max |
5.6-6.8 |
0.2-0.4 |
0.1-0.2 |
V=0.1, Zr=0.18 |
Microstructure-Property Relationships:
Copper which is “the primary alloying element in the 2xxx series alloys” increases the tensile strength, and hardness of aluminum alloys because of the effect of solid solution hardening. It also improve the machinability of alloys by increasing matrix hardness. However, copper reduces the corrosion resistance and the .
The following diagram illustrates the maximum solubility of copper in aluminum (up to 6.5%) (2).
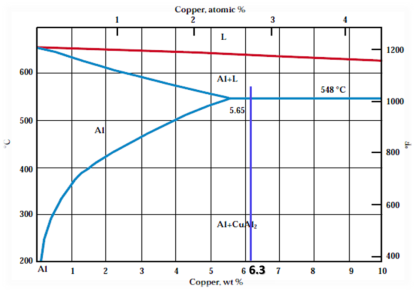
Fig (4): Partial phase diagram for the aluminum-copper system showing the maximum solubility of copper in aluminum
Good solubility of copper in aluminum up to 5.65% at T=548oC (eutectic temperature). Eutectic phase consists of α phase (Al 5.65%Cu) which is ductile and θ phase (CuAl2;52.75%Cu) which is brittle.
5 < % Cu low toughness because (α+θ ) eutectic surrounds grain boundaries of α phase.
> 5% Cu α phase in some cases surrounded by θ phase. 33% Cu so brittle according to high amount of brittle θ phase.
Aluminum-copper alloys are the group of aluminum alloys which are . Where by increasing temperature copper demonstrates increasing solid solubility in aluminum. As sequence significant additional strengthening could be produced and stabilizing of the structure could be achieved (7).
References:
1. Rana R S, Purohit R, Das S. Reviews on the Influences of Alloying Elements on the Microstructure and Mechanical Properties of Aluminium Alloys and Aluminium Alloys composites. International Journal of Scientific and Research Publications, Volume 2, Issue 6, 2012; ISSN 2250-3153.
2. Substances & Technologies. Wrought aluminum-copper alloys (2xxx). Weblog.
Available from:
http://www.substech.com/dokuwiki/doku.php?id=wrought_aluminum-copper_alloys_2xxx
3. Yong Lee C, Hyun Choi D, Bae Lee W, Park SK, Yeon YM, Jung SB. Microstructures and Mechanical Properties of Double-Friction Stir Welded 2219 Al Alloy. Materials Transactions , Vol. 49, No. 4 (2008) pp. 885 to 888.
4. Robinson J. S,. Cudd R. L,. Evans J. T. Creep resistant aluminium alloys and their applications. Materials perspective 2003.
5. The Aluminum Association, Inc. Aluminum Alloy Selection and Applications. 1998; (202) 862-5100.
6. ASM Vol 06 Welding, Brazing, and Soldering.
7. AlcoTec. The Differences Between Heat-Treatable and Non-Heat-Treatable Aluminum Alloys. Weblog.
Available from:
http://www.alcotec.com/us/en/education/knowledge/qa/The-Differences-Between-Heat-Treatable-and-Non-Heat-Treatable-Aluminum-Alloys.cfm
8. Yong Lee1C, Choi1DH, Lee WB, Park SK, Yeon YM, Jung SB. Microstructures and Mechanical Properties of Double-Friction Stir Welded 2219 Al Alloy. 2008;Materials Transactions, Vol. 49, No. 4 (2008) pp. 885 to 888.