Rate of Conversion of Nitrogen Oxide (NOx) into Nitrogen
Â
1.1 Chapter Introduction:
This chapter gives some basic information of the thesis. Firstly, some background information about selective catalytic reduction(SCR) technology, aqueous and dry ammonia, NOx gas and its conversion. Secondly the reason and the motivation for choosing this research project. Then the aim, objectives and methodology is described. Finally, the progress of project, plan and the conclusion is given.
1.2 Background Information:
1.2.1 Selective Catalytic Reduction(SCR) Technology:
SCR technology is an advanced emission control technology used to reduce the quantity of hazardous gases that come out through the exhaust of a diesel engine. It injects a reducing agent into the exhaust of a diesel engine with the help of a catalyst. The reducing agent is usually automotive grade Urea and it is known as a Diesel Exhaust Fluid (DEF). A chemical reaction occurs that converts the Nitrogen Oxides(NOx) into Nitrogen, water and carbon dioxide. SCR technology is made to allow the exhaust gases pass through the reducing agent to take place a chemical reaction in an oxidising atmosphere. The “Selective” word is used because it reduces the amount of NOx using a reducing agent in a catalyst system and the chemical reaction is called as” Reduction” where the DEF is a reducing agent that sets a chemical reaction with NOx and converts it into nitrogen, water and carbon dioxide (CO2)
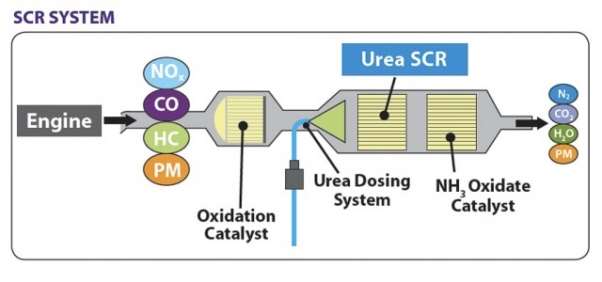
Figure 1 SCR System (Howard, n.d.)
This technology is the most cost effective and fuel efficient technology used to reduce the emission of a diesel engine. The diesel truck engines produced after January,2010 must meet the new EPA standard. SCR reduces NOx emission up to 90% while on the other hand reducing hydrocarbon (HC) and carbon monoxide (CO) emission by 50-90% and particulate matter (PM), 30-50%. More emission reduction for PM can be achieved by attaching this system with the diesel particulate filter. SCR system is widely used in a marine vessel, cargo vessels, ferries, tugboats, large , , and etc.
1.2.2 SCR reducing agent Ammonia:
Some reductants are used in the SCR system such as anhydrous ammonia, aqueous ammonia and urea. These three reductants are widely available in the large quantity. Anhydrous ammonia is highly toxic and difficult to store, but it does not need any conversion, it can be used as it is to operate in the SCR. Anhydrous ammonia is a compressed liquid form of ammonia which does not contain water in it. Anhydrous ammonia is an important industrial cleaner that cleans the harmful bacteria and shines the industrial metal. The large amount of anhydrous ammonia stored requires more caution and safety. Stronger concentration of ammonia can cause burning and some fatal issues. In 1947, a ship filled with solid form of ammonia called as ammonium nitrate exploded in the Galveston Bay of Texas, resulting in number of deaths. Hence using anhydrous ammonia is SCR system is dangerous. (Lepore, n.d.)
Urea is easy and safe to store but it is required to convert into ammonia through thermal decomposition to use as a reducing agent in SCR. Urea is very safe, stable, non-volatile, non-flammable and non- explosive. It can be safely transported, stored and handled. But the disadvantages of Urea are it may cause an extra emission of a nitrogen oxide and carbon monoxide which are harmful gases for environment as well as living organism. It may cause a contamination and corrosion at the point of injection, leakage of Urea may cause a formation of a white precipitate salt and when mixed with a water then the formed Urea solution can cause scaling blockage in SCR system.
Aqueous ammonia which is known as ammonium hydroxide must be hydrolysed to be used and it is comparatively safer to store and transport than anhydrous ammonia. Larger volume of aqueous ammonia will be required as compared to anhydrous ammonia to control the same NOx efficiency. Aqueous ammonia is available in a concentration of 19 percent and 29 percent by weight. For the 29% of aqueous ammonia, the volume required 3.4 times that of anhydrous ammonia and for 19%, the volume required is 5.3 times that of anhydrous ammonia. There are some common things between aqueous ammonia and anhydrous ammonia SCR installation. It has storage tanks, pumps and injection valves. The controlling is same that of anhydrous ammonia. (Salib, n.d.)
1.2.3 NOx conversion:
SCR technology was established in 1970’s and mainly used in stationary sources and still it is measure strategy for the reduction of NO. The high frequency and the ability to react with the NOx selectively to form nitrogen, water and carbon dioxide. When the exhaust gases pass through the SCR system a chemical reaction takes place. The ammonia or other reductant such as urea is injected and mixed with the exhaust gases before entering the catalyst chamber. The following reaction takes place in the conversion of NOx. (Majewski, n.d.)
6NO + 4NH3 → 5N2 + 6H2O
4NO + 4NH3 + O2 → 4N2 + 6H2O
6NO2 + 8NH3 → 7N2 + 12H2O
2NO2 + 4NH3 + O2 → 3N2 + 6H2O
NO + NO2 + 2NH3 → 2N2 + 3H2O
2nd equation shows dominant reaction mechanism. 3rd to 5th reactions above include nitrogen dioxide reactant. The 5th reaction is very fast reaction. NO2 is responsible for the promotion of the low temperature SCR in this reaction. In diesel SCR system, the level of NO2 is increased to enhance the conversion of NOx at low temperature. In some reaction water is produced that shows moisture is always exist in diesel exhaust.
If the NO2 content increases NO level in the feed gas increases. N2O formations are also possible as shown in the reaction below, (Majewski, n.d.)
8 NO2 + 6 NH3 → 7 N2O + 9 H2O
4 NO2 + 4 NH3 + O2 → 4 N2O + 6 H2O
Some Undesirable procedures happening in SCR systems incorporate a few focused, nonselective reactions with oxygen, which is copious in the system. These reactions can either deliver optional emissions or, best case scenario, ineffectively consume ammonia. Partial oxidation of ammonia may deliver nitrous oxide (N2O) or elemental nitrogen, individually. Complete oxidation of ammonia, is shown in the following equation
2NH3 + 2O2 → N2O + 3H2O
4NH3 + 3O2 → 2N2 + 6H2O
4NH3 + 5O2 → 4NO + 6H2O
The SCR procedure requires exact control of the ammonia injection rate. A deficient injection may bring about unsuitably low NOx changes. An injection rate which is too high outcomes in arrival of undesirable ammonia to the climate. These ammonia emissions from SCR system are known as ammonia slip. The ammonia slip increments at higher NH3/NOx proportions. As indicated by the prevailing SCR reaction, 2nd equation the stoichiometric NH3/NOx proportion in the SCR system is around 1. Proportions higher than 1 altogether increment the ammonia slip. By and by, proportions near 0.9 and 1 are utilized, which limit the ammonia slip while as yet giving tasteful NOx changes. The alkali slip diminishes with expanding temperature, while the NOx transformation in a SCR catalyst may either increment or lessening with temperature, depending upon the specific temperature range and catalyst system, as will be examined later.
1.2.4 Diesel engine exhaust system:
The first motivation behind a fumes framework was to securely course fumes gasses from the motor so they can be depleted into the earth, while additionally giving lessening of burning clamor. Fumes gas, nonetheless, contains parts that are hurtful to human wellbeing or potentially the earth. Thus, emanation levels of these fumes gas parts got to be distinctly directed. Since directed discharge levels are regularly much lower than that which can be accomplished through in-barrel control measures, the fumes gas must be dealt with after it leaves the motor. In this manner, while fumes frameworks keep on serving their unique capacities, they have developed into one of the basic components utilized for contamination control and reduction in cutting edge motors.
A fumes framework from a diesel traveler auto is outlined in Figure 1. The fumes framework is regularly associated with the ventilation system, which gathers fumes gasses from the motor chambers’ fumes ports. In light-obligation applications, exhaust systems and diesel particulate channels (DPF) can be put either in the nearby coupled position to the ventilation system (the converter in Figure 1) or in the underfloor position (the particulate channel in Figure 1). The decision of area is controlled by the accessibility of space and the sought temperature profile, with the nearby coupled area giving introduction to the most noteworthy conceivable fumes gas temperatures.
The after-treatment gadgets and their channeling are in some cases alluded to as the “hot end” of the fumes framework, while the suppressors and the tailpipes are the “cold end” of the fumes framework. The hot end channeling may incorporate the “downpipe” or “front pipe” (not present in the design appeared in Figure 1) which associates the ventilation system with the exhaust system, and additionally funneling between the impetus and the particulate channel. The after-treatment framework is associated with the suppressor by the “inside pipe” The presentation to high temperature, alongside different components, for example, quality necessities and synthetic introduction, decide the decision of fumes framework materials.
Debilitate Brakes. Debilitate frameworks may likewise incorporate various segments. Some diesel trucks are outfitted with a “fumes brake”, which utilizes the fumes gas weight for vehicle braking, to facilitate the requests on wheel brakes and increment their life span. By actuating a throttle valve put in the fumes framework when the motor is creating no yield and braking is required, fumes backpressure and accordingly the torque required to pivot the motor is expanded. In motors furnished with a variable geometry turbine (VGT), the turbine vanes might be utilized to throttle the fumes stream rather than a different throttle valve. The adequacy of fumes brakes can be enhanced with an element that holds the fumes valve open constantly (“bleeder brake”). This can be proficient with an actuator that pushes the fumes valve and keeps it open through every one of the four motor strokes.
Exhaust Brakes: – It can be fitted to an assortment of medium-obligation diesel motors including overwhelming get trucks planned to pull moderately substantial trailers. Their application and adequacy is constrained by the greatest weight that the fumes framework segments upstream of the throttling valve can support. Deplete brakes have little effect on fumes framework commotion.
Fumes brakes are just a single approach to utilize the motor to help in vehicle deceleration. Pressure discharge brakes-occasionally alluded to as “motor brakes”- open the fumes valve close to the highest point of the pressure stroke and discharge the packed air into the fumes framework before it can push the cylinder down amid the extension stroke. Pressure discharge brakes are frequently joined straightforwardly into substantial obligation diesel motors, for example, those utilized on Class 8 trucks. They give a fundamentally higher braking impact than fumes brakes without expanding deplete framework backpressure. They are being that as it may, significantly more exorbitant and require exceptional commotion concealment measures to keep away from intemperate fumes framework clamor.
Waste Heat Recovery: – Future fumes frameworks may likewise incorporate fumes gas vitality recuperation frameworks. In the diesel motor, the fumes gas enthalpy speaks to a critical portion of the synthetic vitality of the fuel-up to more than 30%-which is a standout amongst the hugest wellsprings of warm effectiveness misfortune. Debilitate warm recuperation frameworks may run from basic warmth exchangers to complex advances, for example, thermoelectric. An illustration vehicle with a fumes gas warm exchanger is the 2006 Citroen C4 Picasso, where squander fumes warm exchanged by means of the cooling framework is utilized to even more quickly warmth the lodge. Consider went for utilizing thermoelectric generators to deliver power from waste fumes warm in light-and substantial obligation diesel motors has been supported by the US Department of Energy
1.3 Reason and motivation for the research:
Day by day the use of vehicle is increasing rapidly so that the emission of exhaust gases such as CO, NOx and CO2 are also increasing. Ammonia is basically known for being harmful for the environment, additionally adds to the development of particulate matter that has related to unfriendly wellbeing and environmental effects. In industries, the emission of NOx is rapidly increasing and it has become mandatory to control the impact or the emission of such hazardous gases. It may not be decreased but it can be controlled or converted into non-hazardous gases with the help of some reducing agents. Nitrogen oxides (NOx) are an extremely important and essential group of air polluting chemical compound. This clarifies why NOx are critical air toxins and how NOx is formed and react in the air. This notice likewise talks about the standards on which all NOx control and pollution prevention technologies are based; accessible NOx technologies for different combustion sources; and performance and cost of NOx technologies. Following are some toxic effect of NOx:
- It creates photochemical smog
- It causes acid rain and nitrate particulate
- It increases the risk of respiratory conditions and increases the response to allergens
- It causes a formation of ground ozone which is associated with adverse health effect.
This thought motivated to design a technology to convert such gases into non-hazardous gases. Because of the expanding awareness with air quality, stricter emission limits have been formed, which will be much further fixed for off-expressway diesel motors in the coming years. Depending upon the application and the area, distinctive emission limits apply. The most stringent emission regulation is as of now forced by the European Union (EU) and the Environmental Protection Agency (EPA) in USA. Nitrogen Oxide (NOx) and (Particulate Matter) are the main focused pollutant on which the technology is being developed.
An extra test emerges from the fact that, depending upon the application, distinctive test cycles must be utilized for the confirmation of engines. The engines are worked just in chosen regions of the engine map during particular accreditation runs. Just steady state test cycles are utilized for heavy vehicle engine with a power output more prominent than 560 kW. For versatile machinery engine with a power output beneath 560 kW. During the steady state test cycle, the engine is worked at steady speed and load while the emission estimation is directed. After every emission estimation, the working point is changed and the following estimation will be performed. At long last, the general test cycle result is calculated by a weighted sum of all estimations. During the NRTC test, the motor is worked completely transient, with a constant estimation of the emission. Along these lines, the after-treatment system has additionally to have the capacity to perform under transient conditions. This reality prompts to requirement, e.g. highly exact urea dosing. This can be obtained by using the SCR technology
The aim of this research project is to investigate the rate of conversion of Nitrogen Oxide (NOx) into Nitrogen and water when the NOx gas is passed through the reducing agent. Within this aim there are number of objectives:
- Determine the
- Mount the test rig.
- Set temperature and amount of ammonia.
- Adjust the pressure load of the exhaust gas.
- Pass the gas through the test rig.
- Take the 1st default reading without using ammonia and SCR catalytic converter.
- Attach aqueous ammonia and SCR catalytic converter and again take the readings
- Testing Conditions –
- at 25%, 50% and 75% of full load of engine
- The amount of ammonia injection is determined
- Keep the load of engine constant and increase the flow rate of ammonia and take readings on various flow rate.
- Increase the load of engine and repeat the same procedure.
- Compare the readings and plot the graph.
- The same procedure is repeated to get test results of 5 injection rate and determine the optimum level of ammonia injection.
- The above procedure is conducted at exhaust gas temperature in range of 300°C – 500°C and depends on the load of diesel engine.
- Purchase a SCR catalytic converter and ammonium hydroxide (aqueous ammonia).
- Second hand SCR catalytic convertor is available on (For-sale, n.d.)
- Take permission to use the diesel engines and the other required equipment from the college lab.
- Determine the airflow of exhaust gases based on size of the diesel engine
- Airflow measurement with the help of U-tube manometer.
- Measurement of engine speed using RPM indicator.
- Arrange Rotameter to measure the amount of ammonia released in the chamber.
- Mount the experimental rig.
- Solve the errors and find solutions for the problems that will occur during the investigation
Hence the progress of the research concludes that the aqueous ammonia is suitable for the conversion of NOx in the SCR system as it is widely available and safest to store. In addition to it aqueous ammonia is inexpensive.