Recent Applications of Keratin

Abstract
This review discusses the recent applications of keratin and keratin-based materials.

- Keratin-Based Materials
The keratin-based materials are produced from keratin fibers, such as human hair, skin, hooves, feathers, beaks, feet and horns (18).
For biomedical and pharmaceutical purposes, human hair is a preferred major source of keratin for several reasons. First, it is available readily from barber and beauty salons. Also, human hair is less prone to cause undesired allergic or immune reactions in a human. Finally, a derived keratin material is able to be made from the hair of a person for whom the keratin-based material will be used (13).
Animal feathers are also major resources for keratin extraction. Every year, there are 5 million tons of chicken feathers produced from chicken meat as a waste stream (8). Thus, feathers are abundant source of keratin that can be easily obtained.
There are numerous methods to extract the keratin-based material from the keratin sources. One method includes partial oxidization of some disulfide linkages of the keratin with an oxidizing agent, such as peracetic acid, while remaining disulfide linkages are left intact. The partially oxidized hair is powdered and the remaining intact disulfide linkages are cleaved with a reducing agent. An insoluble part of keratin fraction is, then, removed by centrifugation (3, 15). The soluble part, including alpha keratin, is purified and oxidized to reform disulfide linkages between protein backbones (3, 19). The oxidized soluble part is easily dissolved and can form keratin solutions with controlled concentrations (19).
The produced keratin solid can be used in a fibrous form when shredded, in a powder form when finely ground, in a hydrogel or viscoelastic hydrogel when hydrated by adding water, or may be used in certain embodiments (13). These materials are used for biomedical, pharmaceutical, biosorbent, and industrial applications.
- Wound Dressing
The optimum wound dressing protects the injured tissue, maintains moisture while being water permeable, is easy to apply, and delivers effective healing agents to the wounded tissue (15). The keratin-based material acts as a non-antigenic wound healing material (3).
The keratin-based film is appropriate to be used as a wound dressing. The porous sponge matrices of keratin can play an important role in absorbing wound exudates and in maintaining a healthy and moist environment for healing an injury (16). Also, a hydratable keratin solid powder that is also used in a form of a keratin hydrogel when added water is used as a wound dressing (13). These highly absorbent keratin solid fiber and powder provide an extra benefit along with the water absorbency. This benefit includes healing or soothing peptides associated with the keratin (18).
Blanchard et al. (3) tested the keratin power, which would be used to produce a keratin hydrogel when hydrated, as wound healing agent with several donor sites. The sterilized keratin powder is applied on a half of a donor wound site and the other half is treated with a standard treatment. The result shows the halves treated with the keratin powder mature faster and epithelialize more rapidly. Also, the patients with the wounds have significantly less pain with the keratin power treatment (3).
Than et al. (4) conducted a study focusing on the effects of the keratin dressing on chronic wounds of different cases. For one of the studied cases, a minimally exudative wound, which had been existed for 11.5 months, was treated with a matrix dressing produced from freeze-dried keratin protein. This dressing allows the rapid growth of new tissue by reabsorbing into the developed tissue. The wound was healed after 30 weeks (Figure 2). Also, the patient had experienced the repeated leg ulcers; yet, the patient stayed ulcer-free after the treatment (4).
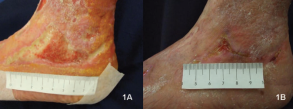
Figure 1 (1A) Ulcer under keratin-derived matrix dressing treatment at Day 0; (1B) Healed ulcer under keratin-derived matrix dressing treatment at Day 99 (4)
- Pharmaceutical
Siller-Jackson et al. (13) and Van Dyke et al. (18) proposed an invention of the keratin material incorporated with nonwoven film, which can be used in several different applications. One of the applications is that the solid keratin with nonwoven film can form a beneficial drug delivery system when it is incorporated with active pharmaceutical agents. These pharmaceutical agents, including the compounds that may allow ion exchange with sulfonic acid groups of keratin, can be formulated as hydrochlorides, polar agents, protein agents, polypeptide agents, and peptide agents (18). Polypeptide agents include both native and recombinant polypeptides (13). Table 1 provides the list of the classes and types of pharmaceutical agents (13, 18).
Table 1 Classes and Types of Pharmaceutical Agents (13, 18)

The invention of Van Dyke et al. (18) suggests that the application of the drug delivery system with solid keratin provides several significant advantages. In this system, the properties of the dosage form of a drug can be determined by the chemical and material properties of the keratin, whereas with most delivery systems, the level of a drug is maintained at a consistent concentration with sustained or controlled release. Also, the nonwoven film drug delivery system is performed in non-aqueous media, which is a distinct advantage because non-water soluble drugs are usually troublesome to formulate into convenient dosage forms. Furthermore, keratin can play a dual role of wound dressing and drug delivery system simultaneously, allowing a less intrusive therapy than separate treatments (18).
- Hemostat
Aboushwareb et al. (7) demonstrated the hemostatic characteristics of the human hair keratin hydrogel with the ability to absorb fluid and bind cells successfully. The experiments evaluate the efficacy of human hair keratin hydrogel in a lethal model of liver injury in a rabbit model, compared to other commercial hemostats. The study proved the efficacy of the keratin biomaterials in arresting hemorrhage and increasing the survivability in a model of liver injury, similarly to the compared commercial products. Also, it was proved that the keratin hydrogel does not produce adverse cell and tissue responses (7).
- Implant Filler
The keratin hydrogel can also be used as an augmentation of soft tissue, including augmentation of vocal chords in order to restore elasticity, and augmentation of breasts, lips, chin, gluteal area, and wrinkled or acne scarred skin in order to improve the appearance of a subject (25).
The biocompatible viscoelastic keratin hydrogel is used as an implant filler (25). Such keratin hydrogel provides a natural-appearing and safe implant for reconstructing or filling the human breast, and other tissues. The implant may be used in several ways. One way is that the solid hydrogel implant precursor is hydrated before placing the filler into an implant envelope. Another way is that tissue expanders are contained in an envelope with the keratin hydrogel. This method allows the implant to absorb the body fluids through the envelope and increasing in a volume at a controlled rate, providing a more convenient and comfortable implant compared to traditional implants (18).
The keratin hydrogel implants are less toxic than the silicone implants, in case of the risk of a leakage. Also, the keratin fillers give more natural appearance and feeling than saline implants do. Additionally, the keratin implants do not require a second invasive procedure to harvest tissue as fat cells do (18).
- Biosorbent
The interest in the use of biomass for the dissolved metal removal from aqueous solutions has been increasing because of the relatively high cost of the traditional water treatment materials, the complex operational set-up, and the safety precautions (9). The keratin-based material can be used as the purification method of natural and waste water resources contaminated with metal (8). The keratin protein fiber is used to purify heavy metal-contaminated water. The wool keratin has been reported to uptake mercury, copper, silver, cadmium, lead, chromium, and aluminum. Also, mohair keratin has been reported to remove copper (9). Khosa and Ullah (10) have recently presented the application of the keratin biopolymer for the removal of arsenic. Also, Saucedo-Rivalcoba et al. (11) have proposed the use of polyurethane-keratin hybrid membranes in order to absorb and remove hexavalent chromium from water.
- Rubber
Hergenrother et al. (12) has proposed the utilization of keratin as a filler in rubber compositions. This use of keratin in conjunction with coupling agents increases dynamic storage modulus (G’) while not affecting the physical properties of the compounds. The keratin filler used is derived from avian feather or feather meal, which has higher bulk density than ground feather. The compounds of the filler are economical and easy to process. Also, these are environmentally friendly because even a small amount of avian feather used will allow the reduced amount of non-renewable fillers, such as carbon black, to be used (12).
The keratin filler used for rubber is beta-keratin-based and water-insoluble. Keratin from feathers is relatively economic, is non-toxic, has a high melting point, is light-weight, and is a biodegradable renewable material. Therefore, the reinforcing keratin filler will help produce sustainable products that uses rubber, such as tires.
- Diapers / Feminine Hygiene Products
The absorbent materials are capable of absorbing body fluids such as urine and menses. Thus, the absorbent materials are included in the products that are used next to the skin. Such materials can be derived from wood pulp, cellulosic fibers, or synthetically produced superabsorbent (13, 18).
An inner core of diapers and feminine hygiene products is designed to absorb water and urine. It is commonly formed with a superabsorbent polymer that is dispersed in a larger amount of less absorbent material. Yet, even the absorbent materials are separated from the skin with at least one layer of materials, the skin contact with such materials have been causing irritation and not beneficial (13, 18).
The keratin-based absorbent or hydratable solid, in forms of powder or hydrogel, is a natural material that can absorb body fluids, and is beneficial with respect to diaper rash. The hydratable keratin solid can be coated either on a layer next to the skin of a subject or on a layer separated from the skin by a water permeable layer (13, 18).
For both diapers and feminine hygiene products, the hydratable keratin solid can be used in an inner absorbent core. The keratin materials may be associated with a nonwoven layer of product, or coated on a layer of a product, or permeated into a layer of a product (13, 18).
- Keratin Hydrolysate
Similar to the keratin-based material sources, keratin hydrolysates are prepared from human hair, wool, animal hair, feathers and horns (21).
The recent method of the keratin hydrolysate production utilizes chicken feathers with Bacillus subtilis (21, 22). Vermelho et al. (21) and Villa et al. (22) have suggested that the useful bacterium for the production is Bacillus subtilis. Villa et al. (22) proposed an effective method that produces a clear hydrolysate (22). Feathers are transformed into keratin peptides and amino acid by peptidases and keratinases, produced by Bacillus subtilis (Figure 2) (22). From this process, the keratin hydrolysates are produced enzymatically (21).
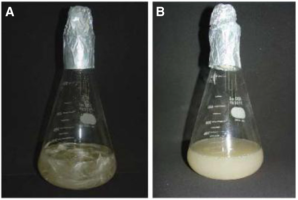
Figure 2 (A) Control: Bacillus subtilis in feather containing medium at Day 0; (B) Growth in feather medium at Day 5 (22)
Such method is also environmental friendly because it recycles and helps reducing the feather waste, which is the byproduct of the poultry industry (27). The keratin hydrolysate is majorly used for cosmetics applications.
- Cosmetics
The keratin hydrolysates can be used in various cosmetic applications, such as hair and skin applications (21).
Villa et al. (22) effectively proved that the enzymatic production of keratin peptides from feathers is significantly affective in hair care products. The keratin peptides improve the hair fiber hydration and seal cuticles in the hair fibers with the hydrolysates, which increase the shine and softness of the hair (22).
Barba et al. (24) conducted a long-term study to find the beneficial effect of the topical application of the wool keratin peptides. The study was performed on undisturbed kin to determine the efficacy of the two keratin peptide samples, one with an aqueous keratin formulation and another with liposome formulation mixed with the aqueous keratin solution. Both of the keratin peptide samples showed very close result with the increase of the hydration of the skin. Also, the treated skin with both samples was resulted with increased skin elasticity (24).
The keratin-based hydrogel is capable of facilitating the regeneration of peripheral nerves. Sierpinski et al. (5) showed that the keratin hydrogel enhances the in vitro activity of Schwann cells, led from the increase of cellular proliferation and migration, and the upregulated gene expression.
References
|
1. |
Rouse, J.G.; Van Dyke, M.E., “A Review of Keratin-Based Biomaterials for Biomedical Applications,” Materials 2010, 3 (2), 999-1014. |
|
|
2. |
Silva, R.; Fabry, B.; Boccaccini, A.R., “Fibrous Protein-Based Hydrogels for Cell Encapsulation,” Biomaterials 2014, 35 (25), 6727-6738. |
|
|
3. |
Blanchard, C.R.; Timmons, S. .; Smith, R.A., “Keratin-Based Hydrogel for Biomedical Applications and Method of Production,” U.S. Patent 6,379,690, April 30, 2002. |
|
|
4. |
Than, M.P.; Smith, R.A.; Hammond, C.; Kelly, R.; Marsh, C.; Maderal, A.D.; Kirsner, R.S., “Keratin-Based Wound Care Products for Treatment of Resistant Vascular Wounds,” J. Clin. Aesthet. Dermatol. 2012, 5(12), 31-35. |
|
|
5. |
Sierpinski, P.; Garrett, J.; Ma, J.; Apel, P.; Klorig, D.; Smith, T.; Koman, L.A.; Atala, A.; Van Dyke, M., “The Use of Keratin Biomaterials Derived from Human Hair for the Promotion of Rapid Regeneration of Peripheral Nerves,” Biomaterials 2008, 29 (1), 118-128. |
|
|
6. |
Apel, P.J.; Garrett, J.P.; Sierpinski, P.; Ma, J.; Atala, A.; Smith, T.L.; Koman, L.A.; Van Dyke, M.E., “Peripheral Nerve Regeneration Using a Keratin-Based Scaffold: Long-Term Functional and Historical Outcomes in a Mouse Model,” J. Hand. Surg.2008, 33A, 1541-1547. |
|
|
7. |
Aboushwareb, T.; Eberli, D.; Ward, C.; Broda, C.; Holcomb, J.; Atala, A.; Van Dyke, M., “A Keratin Biomaterial Gel Hemostat Derived from Human Hair: Evaluation in a Rabbit Model of Lethal Liver Injury,” J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 90B (1), 45-54. |
|
|
8. |
Khosa, M.A.; Ullah, A., “A Sustainable Role of Keratin Biopolymer in Green Chemistry: A Review,” J. Food Processing & Beverages 2013, 1 (1), 8-15. |
|
|
9. |
Kar, P.; Misra, M., “Use of Keratin Fiber for Separation of Heavy Metals from Water,” J. Chem. Technol. Biotechnol. 2004, 79 (11), 1313-1319. |
|
|
10. |
Khosa, M.A.; Ullah, A., “In-situ Modification, Regeneration, and Application of Keratin Biopolymer for Arsenic Removal,” J. Hazard. Mater. 2014, 278, 360-371. |
|
|
11. |
Saucedo-Rivalcoba, V.; Martinez-Hernández, A.L.; Martinez-Barrera, G.; Belascco-Santos, C.; Rivera-Armenta, J.L.; Castaño, V.M., “Removal of Hexavalent Chromium from Water by Polyurethane-Keratin Hybrid Membranes,” Water, Air, Soil Pollut. 2011, 218 (1-4), 557-571. |
|
|
12. |
Hergenrother, W.L.; Shltz, L.L.; Lin, C.J., “Keratin in Rubber Applications,” U.S. Application 14/492,835, January 8, 2015. |
|
|
13. |
Siller-Jackson, A.J.; Van Dyke, M.E.; Timmons, S.F.; Blanchard, C.R.; Smith, R.A., “Keratin-Based Powders and Hydrogel for Pharmaceutical Applications,” U.S. Patent 6,544,548 B1, April 8, 2003. |
|
|
14. |
Kelly, R.J.; Ali, M.A.; Roddick-Lanzilotta, A.D.; Worth, G.; Hassan, M.M.; McLaughlin, J.R.; McKinnon, A.J., “Composite Materials Containing Keratin,” U.S. Patent 7,767,756 B2, August 3, 2010. |
|
|
15. |
Timmons, S.F.; Blanchard, C.R.; Smith, R.A., “Keratin-Based Tissue Engineering Scaffold,” U.S. Patent 6,432,435 B1, August 13, 2002. |
|
|
16. |
Kelly, R.J.; Roddick-Lanzilotta, A.D.; Ali, M.A., “Wound Care Products Containing Keratin,” U.S. Patent 7,732,574 B2, June 8, 2010. |
|
|
17. |
Kelly, R.J.; Worth, G.H.; Roddick-Lanzilotta, A.D.; Rankin, D.A.; Ellis, P.; Mesman, J.R.; Summers, C.G.; Singleton, D.J., “Production of Soluble Keratin Derivatives,” U.S. Patent 7,148,327 B2, December 12, 2006. |
|
|
18. |
Van Dyke, M.E.; Timmons, S.F.; Blanchard, C.R.; Siller-Jackson, A.J.; Smith, R.A., “Absorbent Keratin Wound Dressing,” U.S. Patent 6,270,793 B1, August 7, 2001. |
|
|
19. |
Wu, C.; Li, J.; Wicks, D.; Morgan, S.; Smith, R.A., “Hydratable Keratin Compositions,” U.S. Application 11/920,456, August 11, 2011. |
|
|
20. |
Van Dyke, M.E.; Blanchard, C.R.; Timmons, S.F.; Siller-Jackson, A.J.; Smith, R.A., “Implantable prosthetic or Tissue Expanding Device,” U.S. Patent 6,849,092 B2, February 1, 2005. |
|
|
21. |
Vermelho, A.B.; Villa, A.L.V.; Mazotto de Almeida, A.M.; de Souza Dias, E.P.; dos Santos, E.P., “Keratin Hydrolysates, Process for Their Production and Cosmetic Composition Containing the Same,” U.S. Application 12/666,409, August 5, 2010. |
|
|
22. |
Villa, A.L.V.; Aragão, M.R.S.; Santos, E.P.D.; Mazotto, A.M.; Zingali, R.B.; de Souza, E.P.; Vermelho, A.B., “Feather Keratin Hydrolysates Obtained from Microbial Keratinases: Effect on Hair Fiber,” BMC Biotechnol. 2013, 13 (1), 15 |
|
|
23. |
Weathersby, C.; McMichael, A., “Brazilian Keratin Hair Treatment: A Review,” J. Cosmet. Dermatol. 2013, 12 (2), 144-148. |
|
|
24. |
Barba, C.; Méndez, S.; Roddick-Lanzilotta, A.; Kelly, R.; Parra, J.L.; Coderch, L., “Cosmetic Effectiveness of Topically Applied Hydrolysed Keratin Peptides and Lipids Derived from Wool,” Skin Res. Tech. 2008, 14, 243-248. |
|
|
25. |
Van Dyke, M.E.; Blanchard, C.R.; Timmons, S.F.; Siller-Jackson, A.J.; Smith, R.A., “Water Absorbent Keratin and Gel Formed Therefrom,” U.S. Patent 6,316,598 B1, November 13, 2001. |
|
|
26. |
Misra, M.; Kar, P.; Priyadarshan, G., “Keratin Protein Nano-fiber for Removal of Heavy Metals and Contaminants,” Mat. Res. Soc. Symp. Proc. 2002, 702, |
|
|
27. |
Cedrola, S.M.; de Melo, A.C.; Mazotto, A.M.; Lins, U.; Zingali, R.B.; Rosado, A.S.; Peixoto, R.S.; Vemelho, A.B., “Keratinases and sulfide from Bacillus subtilis SLC to Recycle Feather Waste,” World J. Microbiol. Biotechnol. 2012, 28, 1259-1269. |
|