Synthesis of Ethano Strapped TB’s
Chapter 3 Synthesis of Ethano Strapped TB’s.
3.2 Experimental Section
3.2.1 General procedure for the synthesis of ethano-strapped Tröger’s base.
The methano-strapped TrÓ§ger’s base (4.24 mmol) and 1,2-dibromoethane (1.60 g, 8.48 mmol, 2.0 eq.) were dissolved in N,N-dimethylformamide (5 mL) and lithium carbonate (1.41 g, 19.08 mmol, 4.5 eq.) was added to the mixture which was stirred and heated at 110 °C for 3 days. The mixture was cooled and suspended in ethyl acetate (100 mL) and then washed with water (2 – 25 mL), dried over anhydrous magnesium sulfate, filtered and evaporated to dryness. The crude material was chromatographed (silica gel) to afford the desired ethano-strapped Tröger’s base products.
3.3.5 2,8-Dimethoxy-6H,12H-5,11-ethanodibenzo[b,f][1,5]diazocine X(MHK 02-60)ACT checked NMR
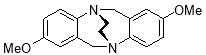
Starting with 2,8-dimethoxy TrÓ§ger’s base X (1.20 g, 4.24 mmol), the crude material obtained upon work-up was chromatographed (silica gel, dichloromethane:ethyl acetate 4:1) to afford X (659 mg, 53%) as an off-white solid. m.p. 185-187 °C (lit.ref 186-189 °C)1. 1H NMR (400 MHz, CDCl3), δ 3.55-3.61 (4H, m, CH2-CH2), 3.68 (6H, s, OCH3), 4.37 (2H, d, J = 17.2 Hz, CH2), 4.55 (2H, d, J = 17.2 Hz, CH2), 6.43 (2H, d, J = 2.8 Hz, ArH), 6.62 (2H, dd, J = 2.8, 8.6 Hz, ArH), 7.07 (2H, d, J = 8.6 Hz, ArH). The data are in agreement with those reported in the literature.1
3.3.4 2,8-Dibromo-6H,12H-5,11-ethanodibenzo[b,f][1,5]diazocine X(MHK 01-120)ACT checked NMR
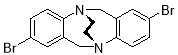
Starting with 2,8-dibromo TrÓ§ger’s base X (1.65 g, 4.24 mmol), the crude material obtained upon work-up was chromatographed (silica gel, dichloromethane) to afford X (750 mg, 45%) as an off-white solid. m.p. 220 °C. 1H NMR (400 MHz, CDCl3) δ 3.47-3.59 (4H, m, CH2-CH2), 4.35 (2H, d, J = 17.4 Hz, CH2), 4.53 (2H, d, J = 17.4 Hz, CH2), 6.96 (2H, d, J = 8.4 Hz, ArH), 7.04 (2H, d, J = 2.1 Hz, ArH), 7.17 (2H, dd, J = 2.1, 8.4 Hz, ArH). The data are in agreement with those reported in the literature.2
3.3.2 6H,12H-5,11-Ethanodibenzo[b,f][1,5]diazocine X(MHK 01-116)ACT checked NMR
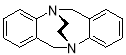
Starting with unsubstituted methano-strapped TrÓ§ger’s base X (942 mg, 4.24 mmol), the crude material obtained upon work-up was chromatographed (silica gel, dichloromethane: ethyl acetate 4:1) to afford X (505 mg, 51%) as an off-white solid. m.p. 169-171 °C (lit.3 174 °C). 1H NMR (400 MHz, CDCl3) δ 3.53-3.68 (4H, m, CH2-CH2), 4.46 (2H, d, J = 17.2 Hz, CH2), 4.61 (2H, d, J = 17.2 Hz, CH2), 6.89-6.96 (4H, m, ArH), 7.03-7.08 (2H, m, ArH), 7.09-13 (2H, m, ArH). The data are in agreement with those reported in the literature.3
5.3.20Di-tert-butyl-3,9-dicarbamate-2,8-dimethyl-6H,12H-5,11-ethanodibenzo[b,f][1,5]diazocine X (MHK-06-108) Sample has a lot of ethyl acetate in it re-run both 1H and 13C NMR
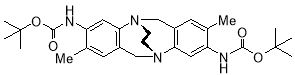
Starting with bis(3,9-tert-butyl-dicarbamate-2,8-dimethyl TrÓ§ger’s base X (5.00 g, 10.42 mmol), the crude material obtained upon work-up was chromatographed (silica gel, dichloromethane:ethyl acetate 1:1) to afford X (2.67 g, 52% with 7% methano strapped as a impurity) as a pale brown solid. m.p. X-Y °C. 1H NMR (400 MHz, CDCl3) δ 1.49 (18H, s, Boc CH3), 2.03 (6H, s, CH3), 3.50-3.60 (4H, m, CH2-CH2), 4.40 (2H, d, J = 17.1 Hz, CH2), 4.48 (2H, d, J = 17.1 Hz, CH2), 6.08 (2H, s, ArH), 6.67 (2H, s, ArH), 7.56 (2H, br s, NH). 13C NMR (100 MHz, CDCl3) δ 17.1, 28.3, 54.9, 58.5, 80.2, 120.3, 128.4, 130.3, 132.0, 134.9, 148.8, 152.9 ppm. FTIR 1049 (m), 1182 (s), 1230 (m), 1709 (s, C=O), 2900 (m), 2972 (m), 3295(bs), cm-1. Anal. Calcd for C28H38N4O4: C 67.99; H 7.74; N 11.33. Found C XX; H XX; N XX %.
3.3.38H,16H-7,15-Ethanodinaphtho[2,1-b][2′,1′-f][1,5]diazocine X(MHK 03-72)ACT checked NMR– contains an impurity ethano strap region should be symmetric
Re-run both 1H and 13C NMR – grow crystals!
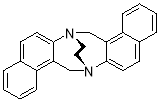
Starting with naphthalene TrÓ§ger’s base X (500 mg, 1.55 mmol), the crude material obtained upon work-up was chromatographed (silica gel, dichloromethane) to afford X (113 mg, 22%) as an off-white solid. m.p. 224-227 °C. 1H NMR (400 MHz, CDCl3) δ 3.75-3.97 (4H, m, CH2-CH2), 4.90 (2H, d, J = 17.5 Hz, CH2), 5.44 (2H, d, J = 17.5 Hz, CH2), 7.27-7.37 (4H, m, ArH), 7.41-7.48 (2H, m, ArH), 7.51 (2H, app. d, J = 8.6 Hz, ArH), 7.67 (2H, app. d, J = 8.0 Hz, ArH), 7.82 (2H, d, J = 8.5 Hz, ArH). 13C NMR (100 MHz, CDCl3) δ 55.2, 55.7, 122.3, 124.4, 126.0, 127.3, 127.5, 128.3, 128.6, 131.5, 132.5, 148.5 ppm. FTIR 828 (s), 927 (s), 1137 (m), 1209 (m), 1469 (m), 2360 (m), 2900 (m), 2959 (m) cm-1. Anal. Calcd for C24H20N2: C 85.68; H 5.99; N 8.33. Found C 85.73; H 5.68; N 8.59%.
3.3.72,8-Dimethanol-6H,12H-5,11-ethanodibenzo[b,f][1,5]diazocine X(MHK 04-50)The spectrum is terrible – there is NO way you can claim to have made this compound – see me
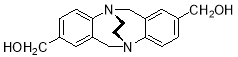
Starting with 2,8-dimethanol TrÓ§ger’s base X (400 mg, 1.42 mmol), the crude material obtained upon work-up was chromatographed (silica gel, dichloromethane: ethyl acetate 1:1) to afford X (134 mg, 32%) as a colourless solid. m.p. X-Y °C (lit.ref A-B °C).2 1H NMR (400 MHz, CDCl3) δ 1.76 (2H, br s, OH), 3.46-3.64 (4H, m, CH2-CH2), 4.43 (2H, d, J = 17.3 Hz, CH2), 4.47 (2H, s, CH2OH), 4.56 (2H, d, J = 17.2 Hz, CH2), 6.89 (2H, app. s, ArH), 7.02 (2H, dd, J = 1.5, 8.1 Hz, ArH), 7.07 (2H, d, J = 8.0 Hz, ArH), 7.26 (2H, s, ArH). 13C NMR (100 MHz, CDCl3) δ 54.6, 59.1, 64.8, 126.1, 127.5, 128.1, 136.7, 137.2, 149.6 ppm. FTIR 750 (s), 884 (s), 1105 (m), 1195 (m), 1328 (m), 1491 (d), 1622 (s), 1701 (s, C=O), 2852 (m), 2893 (bs), 2946 (m) cm-1. Anal. Calcd for C18H20N2O2: C 72.95; H 6.80; N 9.45. Found C XX; H XX; N XX %. See me – is this compound in the literature??(NOT charactrised in letreature) 1. Ishida, Y.; Ito, H.; Mori, D.; Saigo, K., Tetrahedron Lett. 2005, 46, 109-112.
3.3.82-Bromo-8-methyl-6H,12H-5,11-ethanodibenzo[b,f][1,5]diazocine X(MHK-05-18)ACT checked NMR
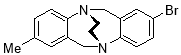
Starting with 2-bromo-8-methyl TrÓ§ger’s base X (1.30 g, 4.12 mmol), the crude material obtained upon work-up was chromatographed (silica gel, dichloromethane) to afford X (1.00 g, 73%) as an off-white solid. m.p. 209-212 °C. 1H NMR (400 MHz, CDCl3) δ 2.19 (3H, s, CH3), 3.47-3.62 (4H, m, CH2-CH2), 4.37 (2H, app. d, J = 17.1 Hz, CH2), 4.53 (1H, d, J = 17.2 Hz, CH2), 4.54 (1H, d, J = 17.2 Hz, CH2), 6.71 (1H, app. s, ArH), 6.86-6.91 (1H, m, ArH), 6.97 (1H, d, J = 8.3 Hz, ArH), 6.99 (1H, d, J = 7.9 Hz, ArH), 7.03 (1H, d, J = 2.1 Hz, ArH), 7.15 (1H, dd, J = 2.1, 8.3 Hz, ArH). 13C NMR (100 MHz, CDCl3) δ 20.7, 54.70, 54.74, 58.7, 59.0, 117.5, 127.7, 128.1, 129.1, 129.7, 130.1, 131.4, 134.4, 136.0, 139.2, 147.2, 149.5 ppm. FTIR 863 (s), 944 (m), 1090 (m), 1219 (s), 1341 (s), 1476 (s), 1518 (s), 2901 (m), 2954 (m) cm-1. Anal. Calcd for C17H17BrN2: C 62.02; H 5.20; N 8.51. Found C 62.29; H 5.12; N 8.68%.
3.3.92-Bromo-8-methoxyl-6H,12H-5,11-ethanodibenzo[b,f][1,5]diazocine X(MHK-04-34)ACT checked NMRReplot 13C with expansions of all picked peaks and show ACTMay need to re-run 13C with more scans – not sure about some peaks
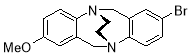
Starting with 2-bromo-8-methoxy TrÓ§ger’s base X (500 mg, 1.51 mmol), the crude material obtained upon work-up was chromatographed (silica gel, dichloromethane:ethyl acetate 2:1) to afford X (180 mg, 35%) as a pale brown solid. m.p. 156-157 °C. 1H NMR (400 MHz, CDCl3) 3.50-3.60 (4H, m, CH2-CH2), 3.68 (3H, s, OCH3), 4.34 (1H, d, J = 17.3 Hz, CH2), 4.37 (1H, d, J = 17.2 Hz, CH2), 4.52 (1H, d, J 17.3 Hz, CH2), 4.54 (1H, d, J = 17.2 Hz, CH2), 6.42 (1H, d, J = 2.9 Hz, ArH), 6.63 (1H, dd, J = 2.9, 8.6 Hz, ArH), 6.98 (1H, d, J = 8.4 Hz, ArH), 7.01-7.06 (2H, m, ArH), 7.16 (1H, dd, J = 2.0, 8.4 Hz, ArH). 13C NMR (100 MHz, CDCl3) δ 54.7, 54.8, 55.2, 58.8, 59.2, 112.8, 113.3, 117.6, 128.8, 129.7, 130.2, 131.5, 137.5, 139.0, 149.4, 156.6, 165.6 ppm. FTIR 805 (m), 846 (m), 1025 (s), 1066 (s), 1278 (s), 1469 (s), 1487 (m), 1594 (m), 2359 (m), 2900 (m) cm-1. Anal. Calcd for C17H17BrN2O: C 59.14; H 4.96; N 8.11. Found C 59.26; H 4.72; N 8.08%.
3.3.102-Ethoxycarbonyl-4,8-dimethyl-6H,12H-5,11-ethanodibenzo[b,f][1,5]diazocine X(MHK-04-30)ACT checked 1H NMR – NEED 13C NMR
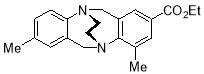
Starting with 2-ethoxycarbonyl-4,8-dimethyl TrÓ§ger’s base X (500 mg, 1.55 mmol), the crude material obtained upon work-up was chromatographed (silica gel, dichloromethane:ethyl acetate 4:1) to afford X (88 mg, 17%) as a pale yellow solid. m.p. 182-185 °C. 1H NMR (400 MHz, CDCl3) δ 1.32 (3H, t, J = 7.1 Hz, CH3), 2.17 (3H, s, CH3), 2.38 (3H, s, CH3), 3.54-3.66 (4H, m, CH2-CH2), 4.20-4.33 (3H, m, CH2), 4.49 (1H, d, J = 17.4 Hz, CH2), 4.50 (1H, d, J = 17.2 Hz, CH2), 4.60 (1H, d, J = 17.2 Hz, CH2), 6.69 (1H, app. s, ArH), 6.85-6.89 (1H, m, ArH), 7.02-7.09 (1H, m, ArH), 7.46 (1H, app. s, ArH), 7.65 (1H, app. s, ArH). 13C NMR (100 MHz, CDCl3) δ 14.3, 17.8, 20.7, 54.7, 55.4, 55.4, 59.3, 60.6, 126.1, 127.8, 128.1, 129.0, 130.0, 134.3, 135.5, 136.5, 137.0, 147.2, 152.8, 166.5 ppm. FTIR 776 (s), 833 (s), 905 (m), 1025 (s), 1215 (s), 1293 (s), 1497 (s), 1709 (s, C=O), 2900 (m) cm-1. Anal. Calcd for C21H24N2O2: C 74.97; H 7.19; N 8.33. Found C 74.72; H 7.25; N 8.41 %.
2.3.118-Bromo-2-ethoxycarbonyl-4-methyl-6H,12H-5,11-ethanodibenzo[b,f][1,5]diazocine X (MHK-05-22)ACT checked NMRNeed to re-run 13C with more scans – insufficient aryl peakssections of 1H MR should go in thessi with discussion – see ACT
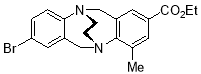
Starting with 8-bromo-2-ethoxycarbonyl-4-methyl TrÓ§ger’s base X (5.50 g, 14.21 mmol), the crude material obtained upon work-up was chromatographed (silica gel, dichloromethane:ethyl acetate 3:1) to afford X (1.70 mg, 30%) as pale yellow solid. m.p. 196 °C. 1H NMR (400 MHz, CDCl3) δ 1.33 (3H, t, J = 7.1 Hz, CH3), 2.36 (3H, s, CH3), 3.54-3.64 (4H, m, CH2-CH2), 4.21 (1H, d, J = 17.5 Hz, CH2), 4.24-4.34 (2H, 2 x overlapping q, J = 7.1 Hz, CH2-CH3), 4.47 (1H, d, J = 17.3 Hz, CH2), 4.49 (1H, d, J = 17.4 Hz, CH2), 4.57 (1H, d, J = 17.3 Hz, CH2), 6.97 (1H, d, J = 8.4 Hz, ArH), 7.01 (1H, d, J = 2.2 Hz, ArH), 7.15 (1H, dd, J = 2.2, 8.4 Hz, ArH), 7.44-7.46 (1H, m, ArH), 7.65-7.67 (1H, m, ArH). 13C NMR (100 MHz, CDCl3) δ 14.3, 17.8, 54.6, 55.0, 55.2, 59.1, 60.7, 117.6, 126.3, 128.0, 129.9, 130.2, 130.3, 131.2, 135.6, 136.6, 139.1, 149.2, 152.3, 166.4 ppm. FTIR 827 (s), 927 (s), 1023 (m), 1150 (s), 1387 (s), 1412 (m), 11470 (s), 1704 (s, C=O), 2360 (m), 2900 (m) cm-1. Anal. Calcd for C20H21BrN2O2: C 59.86; H 5.27; N 6.98. Found C 59.76; H 5.19; N 7.21%.
3.3.121,4,8-Trimethyl-2-nitro-6H,12H-5,11-ethanodibenzo[b,f][1,5]diazocine X(MHK-02-10)Need 1H and 13C NMR where are these???!!!???
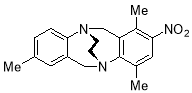
Starting with 1,4,8-trimethyl-2-nitro- TrÓ§ger’s base Y (500 mg, 1.62 mmol), the crude material obtained upon work-up was chromatographed (silica gel, dichloromethane:ethyl acetate:hexane 4:1:1) to afford X (153 mg, 29%) as a yellow solid. m.p. 138-141 °C. 1H NMR (400 MHz, CDCl3) δ 2.20 (6H, s, CH3), 2.36 (3H, s, CH3), 3.54-3.64 (4H, m, CH2-CH2), 4.33 (1H, d, J = 17.5 Hz, CH2), 4.36 (1H, d, J = 17.6, CH2), 4.50 (1H, d, J = 17.5 Hz, CH2), 4.65 (1H, d, J = 17.6 Hz, CH2), 6.75 (1H, app. s, ArH), 6.89 (1H, app.d, J = 7.9 Hz, ArH), 7.01 (1H, d, J = 8.0 Hz, ArH), 7.40 (1H, s, ArH). 13C NMR (100 MHz, CDCl3) δ 14.7, 17.8, 20.7, 54.1, 54.9, 55.7, 57.2, 124.1, 125.0, 127.9, 128.3, 128.6, 128.8, 134.2, 134.6, 136.5, 136.8, 147.2, 152.4 ppm. FTIR 819 (m), 1053 (s), 1185 (m), 1280 (s), 1369 (m), 1497 (m), 2359 (m), 2900 (m), 2987 (m) cm-1. Anal. Calcd for C19H21N3O2: C 70.57; H 6.55; N 12.99. Found C 70.52; H 6.28; N 12.69%.
3.3.142,8-Dimethyl-4-nitro-6H,12H-5,11-ethanodibenzo[b,f][1,5]diazocine X(MHK-02-10, MHK04-66 ChromA1)re-run 1H and 13 Spectra
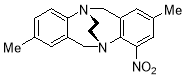
Starting with 2,8-dimethyl-4-nitro-Tröger’s base X (500 mg, 1.69 mmol) and 1with heating for 5 days,the crude material obtained upon work-up was chromatographed (silica gel, dichloromethane: ethyl acetate 10:1) to afford Y (120 mg, 23%) as a yellow solid. m.p. 168-170 °C. 1H NMR (400 MHz, CDCl3) δ 2.20 (3H, s, CH3), 2.21 (3H, s, CH3), 3.42-3.63 (4H, m, CH2-CH2), 4.44 (1H, d, J = 17.6 Hz, CH2), 4.50 (2H, app. s, CH2), 4.62 (1H, d J = 17.6 Hz, CH2), 6.79 (1H, app. s, ArH), 6.87-6.94 (2H, m, ArH), 7.02 (1H, d, J = 8.0 Hz, ArH), 7.11 (1H, app. s, ArH). 13C NMR (100 MHz, CDCl3) δ 20.5, 20.7, 54.4, 56.0, 58.0, 59.4, 122.0, 127.5, 128.1, 129.4, 132.2, 134.6, 135.4, 136.0, 139.4, 140.8, 146.9, 150.5 ppm. FTIR 836 (m), 884 (m), 1021 (m), 1171 (s), 1371 (m), 1521 (s), 2910 (m), cm-1. Anal. Calcd for C18H19N3O2: C 69.88; H 6.19; N 13.58. Found C 69.67; H 6.24; N 13.43%.
References
1.Hamada, Y.; Mukai, S., Tetrahedron: Asymmetry 1996, 7, 2671-2674.
2.Ishida, Y.; Ito, H.; Mori, D.; Saigo, K., Tetrahedron Lett. 2005, 46, 109-112.
3.Faroughi, M.; Try, A. C.; Turner, P., Acta Crystallogr., Sect. E: Struct. Rep. Online 2008, 64, o458.