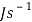Thermodynamics and Fluid Mechanics: Coffee Filter Machine
Â
Background/Introduction
In this assignment they are tasked to explain how an electric coffee filter machine works and its scientific process. they are also tasked to calculate the amount of power it uses and energy it needs to keep that coffee warm and its efficiency when it’s keeping it warm, which is important because if the coffee isn’t very efficient and uses a lot of energy or a lot of heat can escape then it could lead to high energy prices just to keep coffee warm. Once the efficiency has been calculated they also need to find out how it can be improved further, they are required to at least focus on one element to improve its efficiency.
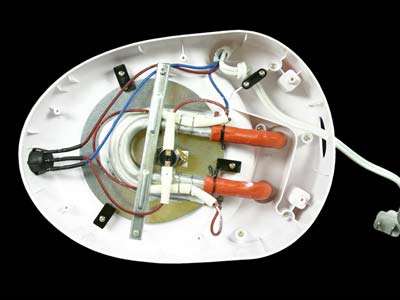
This coffee machine works by holding the water you put in it in a reservoir, that water then goes down a hole in the reservoir and into an orange tube. This tube is used to carry the cold/room temperature water to the aluminium tubing which conducts the heat from the heating element through the warming plate which consists of a heat conductive grease that helps heat the water up. The heating element does this when its coiled wire heats up as electricity runs through it. This heating element has two purposes, one of which is initially heating the water up and its second purpose comes in a later process. The aluminium tube is connected to the white tube which carries that hot water up to the shower head. This white tube doesn’t consist of a pump to carry the water up, instead the bubbles from the boiling water rise in the tube and carry the water up to the shower head and the machine’s one way valve allows the water to go up the tube without coming back down and into the reservoir. Once the hot water reaches the shower head, it gets evenly sprayed on to the coffee grounds, picks up the caffeol and gets poured into the coffee pot. Once the coffee has been brewed the second purpose of the heating element is keeping the coffee warm which has many advantages one of which is to keep it warm for other people in the house after you brewed your own. [1]
These coffee machines are very reliable but there is always a possibility of failure, although a few have easy fixes. One way the coffee machine can fail is when the switch or even its power cord stop working which can be repaired by a professional although can sometimes be expensive so it would be more cost effective to buy a new machine. Another way is that the one-way valve can get clogged up letting the water back down the tube or simply not letting the water through at all. An easy fix for this is by simply scooping out any debris with a small object such as a toothpick. The tubes can also get clogged up. A way to fix this would be by running a cycle with vinegar and then running 2 cycles of clean water to rid of any vinegar left in the machine.
Fig 1. [2]
Experiment Analysis
To collect their results, they had to weigh a few things. They weighed the jug when it was empty, the jug when it was full and calculated how much the water weighed. They did their experiment by letting the coffee machine heat the water up until its finished, they then took the jug filled with hot water and measured the temperature change every 2 minutes. Here are their results for 12 cups:
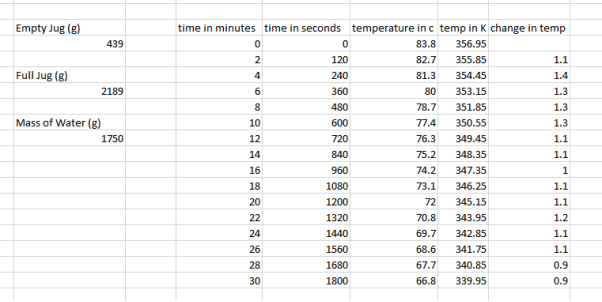
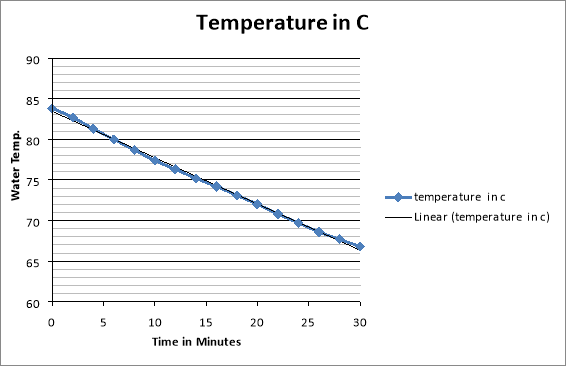
They decided to take the jug out of the coffee machine when measuring their results because then when they are to calculate the energy needed to keep the coffee warm, they minimise the chances of under calculating as the coffee machine only partially covers the jug so not as much heat would escape, taking the jug out allows us to check the maximum amount of heat that can escape over certain intervals. They see that the water started off at 83 degrees Celsius and went down to 66.8 degrees Celsius with the temperature changing roughly between 1-1.4 degrees Celsius every 2 minutes. For the experiment they used a scale for weighing the empty jug and the jug when it was filled with water. They also used an electronic thermometer to accurately measure water temperatures. A coffee machine was also used as the experiment couldn’t be done without it to heat the water up. For 10, 8, 6 and 4 cups the method the exact same although less water was used, here are the results:
10 Cups Results:
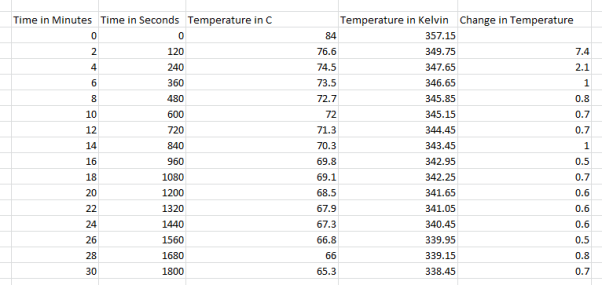
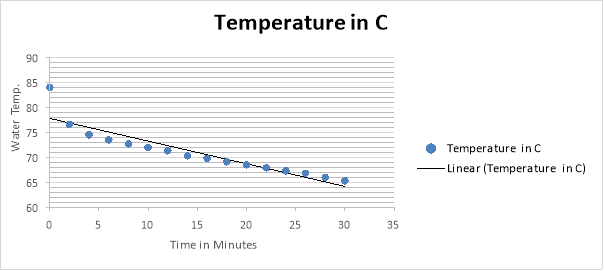
As you can see in the 10 cps results, there is a dramatic change in temperature in the first 2 minutes in comparison to the 12 cups results.
8 Cups Results:
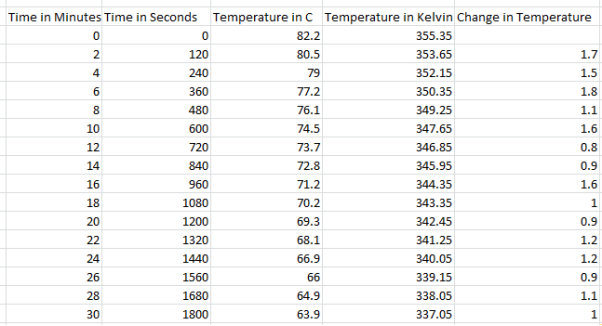
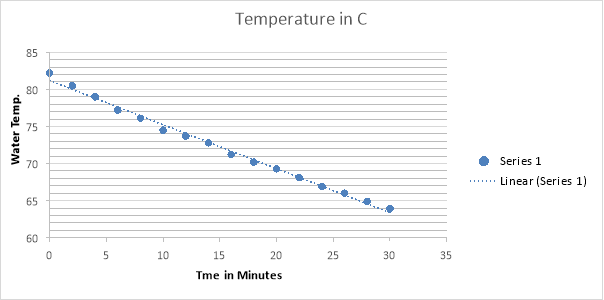
6 Cups Results:
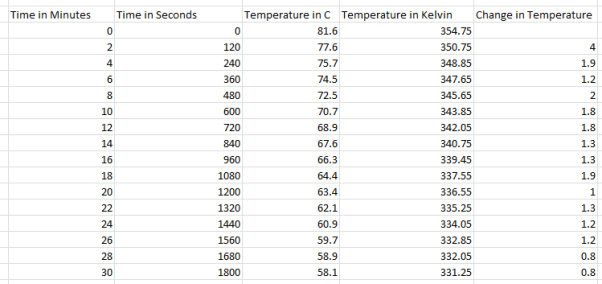
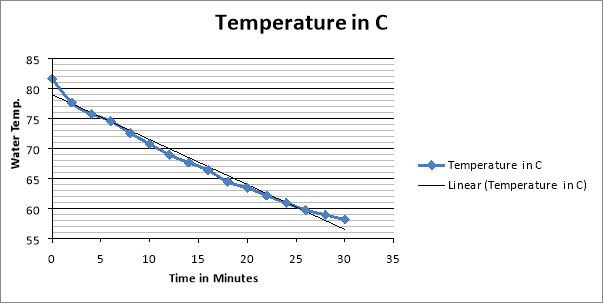
4 Cups Results:
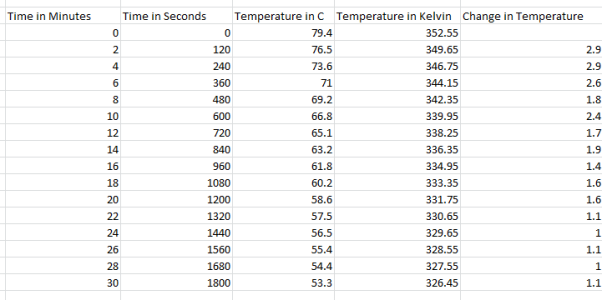
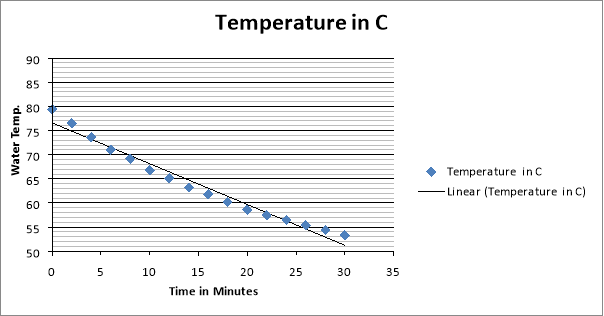
They can use the results from this experiment to calculate heat loss and the rate of heat loss. To calculate the heat loss (Q – Units: -Joules) they take the mass of the water and multiply it by the specific heat capacity and then multiply it by the change in temperature (Q= m*Cp*∆T). Then they did the rate of the heat loss (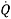 – units: –
– units: – 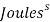 ) which is mass of the water multiplied by the specific and then multiply it by the change of temperature and then divide it by the change of time (
) which is mass of the water multiplied by the specific and then multiply it by the change of temperature and then divide it by the change of time (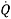 = m*Cp*
= m*Cp*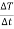 ) Below are the calculations for each of the experiments:
) Below are the calculations for each of the experiments:
|
Amount of Water |
Heat Loss (Q) |
Rate of Heat Loss ( |
|
12 Cups |
124563.25 J |
69.20 |
|
10 Cups |
136682.90 J |
63.38 |
|
8 Cups |
89264.75 J |
49.59 |
|
6 Cups |
89145.42 J |
49.53 |
|
4 Cups |
71119.88 J |
39.51 |
Â
|
Amount of Water |
Mass of Water |
|
12 Cups |
1.75 kg |
|
10 Cups |
1.46 kg |
|
8 Cups |
1.17 kg |
|
6 Cups |
0.91 kg |
|
4 Cups |
0.65 kg |
Costs:
In the UK it currently costs 0.01174 pence per Watt per hour. To find how much it costs per second they have to divide that number by 3600.
0.01174/3600 = 3.261111111 x 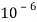 Js-1
Js-1
To calculate how much it costs to keep the coffee hot they use their new cost per Watt per second and multiply it by the rate of heat loss (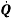 ) and then multiply it by 3600 to get the cost per hour to keep the coffee hot.
) and then multiply it by 3600 to get the cost per hour to keep the coffee hot.
|
Amount of Water |
|
Cost (Pence per Wh) |
|
12 Cups |
69.20 |
0.8124048 |
|
10 Cups |
63.38 |
0.7440812 |
|
8 Cups |
49.59 |
0.5821866 |
|
6 Cups |
49.53 |
0.5814822 |
|
4 Cups |
39.51 |
0.4638474 |
Discussion
Because the coffee machine is an open and transient system, the coffee jug and reservoir is constantly losing heat the coffee machine needs to have good thermal insulation to prevent heat from escaping. One improvement that could’ve been made to the results is use the equation for thermal resistance to calculate the rate of heat loss. By calculating the thermal resistance of the glass jug we can calculate how much heat escapes from it more accurately. The thermodynamics heat processes are conduction, convection and radiation. Mentioned in the background is how the heating element heats up the water through the warming plate, this happens by the process of conduction which is moving heat from a warm location to a cold location, the heat plate and from the heat plate to the aluminium tubing which heats the water up. In the analysis results, specifically for the 10 Cups, there was an anomaly in the first bit of data, the difference in temperature was very high in comparison to the others and this was due to human error. In the first 2 minutes the stopwatch might’ve not been started at the correct time or there might’ve been a problem when the temperature was being measured. One improvement that could’ve been made to the coffee machine to increase its efficiency could be to redesign the coffee jug and manufacture it out of insulated metal to decrease heat loss but with a piece of glass down the middle of it so that you can still see how much coffee is left in the jug, doing this will ensure that less heat escapes the coffee jug, having a big impact on the cost to keep the coffee warm.
Â
References:
[1] Brain, M., & Toothman, J. (2006, November 29). How coffee makers work. Retrieved February 2, 2017, from
[2] Retrieved February 2, 2017, from
An early cam was built into water-driven from the 3rd century BC. The camshaft was later described in () by in 1206. He employed it as part of his automata, water-raising machines, and such as the . The cam and camshaft later appeared in European mechanism