Thalamic Glutamate as a Marker of Global Brain Pathology -MS
|
|
Author contributions:
LP – design & conceptualisation of the study, analysis and interpretation of data, drafting the manuscript for intellectual content.
JR – design & conceptualisation of the study, data collection, analysis and interpretation of data, drafting the manuscript for intellectual content.
IRB – analysis and interpretation of data, revising the manuscript for intellectual content.
GS – analysis and interpretation of data
KZ – data collection
RN – design & conceptualisation of the study, analysis and interpretation of data, drafting the manuscript for intellectual content.[LP1]
Disclosures:
LP – no disclosures.
IRV – no disclosures.
GS – no disclosures.
KZ – no disclosures.
RN – Bayer, Biogen, Genzyme, Merck Serono, Roche – honorarium for speaking, advisory boards. Biogen, Genzyme, Novartis – funds for organising education, staff. Biogen, Novartis – Principal investigator.[LP2]
Multiple sclerosis
Multiple sclerosis (MS) is characterised by demyelination and variable degrees of axonal loss and gliosis. People with MS (pwMS) present with sensory disturbances, spasticity, fatigue, ataxia, pain and urinary dysfunction1. The most common form of MS is relapsing-remitting and 85% of pwMS initially present with it, with most eventually progress to a secondary, progressive phase2. Without adequate treatment, 25% of pwMS become wheelchair-bound3.
Charcot was the first to describe the inflammatory demyelinating plaque as a hallmark of MS in the late 19th century4. While white matter lesions (WML) contribute to disability5,6, they are likely not its only drive. Recent evidence supports the concept that grey matter lesions (GML) and atrophy are likely contributors to disability7,8. Furthermore, recent studies have looked at diffuse axonal loss and support the notion that this process drives long-term disability, due to a combination of focal inflammation and cortical damage driven by meningeal inflammation9-13.
Large clinical trials in MS infrequently correlate the effect of therapies with brain lesion volumes and atrophy. This is due to the fact that as of today, no automated software exists which is able to consistently calculate WMLs14 and GMLs are grossly underestimated as they are not readily visible on MRI15,16. Lastly, brain atrophy is hard to quantify, can only be measured longitudinally and is subject to non-tissue related (pseudo-atrophy) volume loss subsequent to disease modifying treatment17,18. There is an unmet need for a simple biomarker that can act as a surrogate for neuronal damage in MS for use in observational and interventional studies.
Natalizumab
Natalizumab (Tysabri) is a disease-modifying treatment given intravenously as a monthly infusion19. In the UK it is licensed as a second-line treatment for severe, rapidly evolving, relapsing-remitting MS. It is directed against the α4 subunit of integrin on lymphocytes and acts as an immune-modulator by inhibiting their migration to the brain20,21. Compared to placebo, it has been shown to reduce relapse rate by 68%. Furthermore, it reduced the risk of disability progression by 42%, defined as a change in EDSS score sustained for 24 weeks21.
Magnetic resonance spectroscopy
Magnetic resonance spectroscopy (MRS) is a non-invasive MRI sequence that allows identification and quantification of in vivo metabolites present in a small, preselected brain region. Proton nuclei (1H) are most commonly used in studies of the human brain due to their abundance and high sensitivity. MRS sequences distinguish between different metabolites by measuring the frequency at which 1H nuclei flip, which is in turn dependent on the molecular group carrying the hydrogen atom22. Measuring these metabolic changes allows researchers to gain an insight into changes at a cellular and molecular level in the brain, which cannot be acquired using conventional MRI techniques23.
The thalamus is a subcortical hub, with multiple reciprocal connections to both white matter tracts and cortical grey matter24. Previous studies evidenced the fact that it is sensitive to pathology occurring in other brain regions25. We speculated that by using the thalamus as our region of interest (ROI), investigated metabolites would give a measure of global neuronal damage.
Aims
We investigated thalamic MRS as a biomarker for global brain neuronal damage in MS by comparing baseline metabolite concentrations between pwMS and HCs. Metabolites that were found to be statistically significantly different between these two groups at baseline were investigated further. To additionally support using MRS imaging as a surrogate for global central nervous system pathology, we investigated the correlation between these metabolite concentrations in pwMS and total lesion volume. In order to investigate whether thalamic MRS can be used to monitor treatment response, we measured changes in their concentration following treatment with the disease-modifying drug natalizumab.
Population
Participants aged 21-65 underwent inclusion criteria screening. For the pwMS group, this included satisfying the McDonald criteria 2010, having highly active MS and having been scheduled to initiate natalizumab treatment as part of routine NHS Case. Following ethics approval and written informed consent from participants, 17 pwMS and 12 HCs were recruited to the study.
HCs underwent an MRI baseline scan while pwMS underwent a scan at baseline, and follow-up scans at 10 and 56 weeks after initiation of natalizumab treatment.
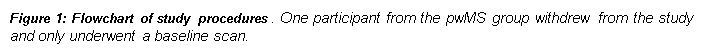
Acquisition of MRS data
All experiments were carried out in the same Siemens 3T Magnetom Verio with a 32-channel receiver head coil[LP4], used to acquire combined MRI and 1H-MRS scans. A magnetisation-prepared rapid gradient-echo sequence (MPRAGE) was used to obtain high-definition T1 weighted scans with the following parameters: (repetition time (TR)= 2300s;echo time (TE)= 3ms; inversion time (TI)= 900; 160 sagittal sections; slice thickness 1.0mm; in-plane resolution of 1x1mm2 . A single voxel was placed over the left thalamus. In order to acquire the single-voxel scans, a Point-RESolved Spectroscopy sequence (PRESS) was used which had variable power and optimized relaxation delays (VAPOR) water suppression (TR/TE, 2000/30ms) on a single 15-mm slab. This was aligned to the T1 sequence sections (Figure 2). Four reference transients were used to align the data. The average of 96 transients was used for water suppressed spectra. The volume of interest was 15x15x15mm, voxel size was 3.4mL. These parameters were also used to acquire reference MRS datasets without water suppression. This was done to obtain an internal water reference, which was used to scale metabolite signals. Double inversion recovery pulse and phase sensitive inversion recovery sequences were also acquired.

Lesion volumes
White and grey matter lesions were identified on 160-slice T1 scans with co-registered double inversion recovery sequences. Lesions were manually segmented in T1 space using the Imperial College software ImSeg. The images obtained by this process [LP5]were used to derive proportions of grey matter, white matter and total lesion volumes. T1, double inversion recovery pulse and phase sensitive inversion recovery sequences were used to check for presence of lesions in the thalamus.
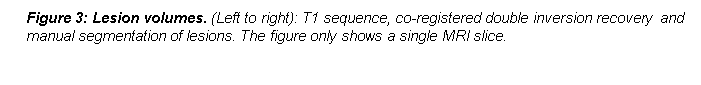
Data processing
T1 and spectroscopy data were initially obtained from scans in dicom format (dcm). A modified MATLAB (v.2015b) script was used to convert the T1 scans into nifti format (nii), the single voxel spectroscopy scans into rda format (rda) and to generate mask files in rda format.
LCModel (v.6.3-1K) was run by using a second modified MATLAB script, in order to obtain spectroscopy data from 0.2-4.0 ppm. The software is a user-independent fitting routine that works by superimposing spectra obtained in vivo with high-resolution model spectra. It is an accurate and reliable method to quantify MRS data with short echo times (ET≤30ms)28,29.
Partial volume corrections to explain different concentrations of water in the grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) were conducted by converting T1 sequences from dicom to nifti format, and segmenting the obtained images using MATLAB’s SPM8 toolbox. This allowed scaling metabolite concentrations obtained from PRESS sequence with water-suppression, to the water’s internal reference signal from the unsuppressed water PRESS-sequence.
The segmentation was used to calculate voxel proportions of GM, WM and CSF, which are in turn needed to obtain the water concentration (WCONC) value from the unsuppressed water reference signal used to estimate absolute concentrations of metabolites. Total WCONC values for each voxel were computed in accordance with Section 10.2.2.3 of the LCModel manual29. Eddy-current correction was performed by using LCModel. Relaxation effects were not corrected for, and therefore reported metabolite concentrations will differ from actual ones by an unknown factor. The latter is likely to be negligible, as all reported concentrations will deviate from actual concentrations by this same, unknown factor. As per LCModel’s manual, metabolite concentrations were multiplied by a factor of 1.04, which amounts to the specific gravity of brain tissue29, and were reported in mmol/L (mM).
Eddy-current correction was performed by using LCModel. Relaxation effects were not corrected for, and therefore reported metabolite concentrations will differ from actual ones by an unknown factor. The latter is likely to be negligible, as all reported concentrations will deviate from actual concentrations by this same, unknown factor. As per LCModel’s manual, metabolite concentrations were multiplied by a factor of 1.04, which amounts to the specific gravity of brain tissue29, and were reported in mmol/L (mM).
Data exclusion
A heat map (Figure 4, right side) was created in order to check for voxel placement by using FSL view v.3.2.0. T1 sequences and mask files were reoriented to match the Montreal Neurological Institute standard template, followed by brain extraction from the surrounding tissue. T1 sequences and mask files were registered to standard space using the Montreal Neurological Institute template, which consists of 152 averaged brain T1 scans of 2mm resolution. The heat map is a depiction of each voxel mask overlaid onto the che2better template for T1 sequences taken from the mricron software.[LP6] No MRS spectra were removed from the analysis owing to minimal inter-scan variability. Spectra generated by LCModel were checked for overall data quality in accordance with the software’s instruction manual29. 2 baseline HC and 2 pwMS spectra were excluded from data analysis (Table 1).

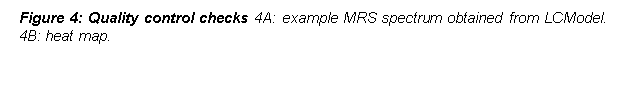
For a metabolite to be investigated, it had to be relevant to MS pathology as evidenced by previous studies, as well as to demonstrate sufficient data quality, measured by having Cramér -Rao lower bounds ratio of <15% in >75% of individual scans. Five metabolites were investigated: choline-containing compounds (Cho), glutamate (Glu), myo-inositol (Ins), total creatine (tCr) and total n-acetylaspartate (tNAA) (Table 1). In a given subject’s scan, metabolite concentrations with a Cramér-Rao lower bounds (CRLB) value of ≥15% were excluded from data analysis, as per LCModel’s manual of instructions. Concentrations exceeding 2 standard deviations (2SD) out with the group mean were also excluded.
|
QCa for entire spectra |
QC for individual metabolites |
||||||
|
Participant group |
Before spectra QC (n) |
After (n) |
Metabolites (marker of)6 |
Participant group |
Before |
After 1st QCf (n) |
After 2nd QCg (n) |
|
HCsb |
12 |
10 |
Cho1 (membrane turnover) |
HCs |
10 |
9 |
9 |
|
pwMS BLc |
17 |
15 |
pwMS BL |
15 |
12 |
12 |
|
|
pwMS 10wd |
16 |
16 |
pwMS 10w |
16 |
16 |
16 |
|
|
pwMS 56we |
16 |
16 |
pwMS 56w |
16 |
15 |
15 |
|
|
Glu2 (metabolism and |
HCs |
10 |
6 |
6 |
|||
|
pwMS BL |
15 |
9 |
8 |
||||
|
pwMS 10w |
16 |
14 |
14 |
||||
|
pwMS 56w |
16 |
15 |
14 |
||||
|
Ins3 (glial marker) |
HCs |
10 |
7 |
7 |
|||
|
pwMS BL |
15 |
14 |
14 |
||||
|
pwMS 10w |
16 |
15 |
14 |
||||
|
pwMS 56w |
16 |
15 |
15 |
||||
|
tCr4 (metabolic activity) |
HCs |
10 |
10 |
10 |
|||
|
pwMS BL |
15 |
15 |
14 |
||||
|
pwMS 10w |
16 |
16 |
15 |
||||
|
pwMS 56w |
16 |
16 |
16 |
||||
|
tNAA5 (neuronal loss, mitochondrial activity) |
HCs |
10 |
10 |
9 |
|||
|
pwMS BL |
15 |
15 |
14 |
||||
|
pwMS 10w |
16 |
16 |
16 |
||||
|
pwMS 56w |
16 |
16 |
15 |
||||
|
|
|||||||
Statistical analysis
Prism GraphPad (v.7) and IBM SPSS Statistics 24 software were used to conduct statistical analysis. Participant demographics results are reported as mean and standard deviation (SD). Metabolite concentrations are reported as mean, standard error of measurement (SEM) and 95% confidence intervals. Parametric tests were used after testing for normal distribution of the data. Unpaired t-tests were used to compare metabolites between pwMS and HCs cross-sectionally. Pearson’s coefficient was used to correlate between metabolite concentrations and bilateral lesion volumes. A linear mixed model was used to quantify longitudinal changes in metabolite concentrations in pwMS.
MRS data were obtained from 17 pwMS (mean age (SD) was 41.6 (10.6), range 21-58 years) and 12 HCs (mean age (SD) was 41.9 (8.3), range 29-61 years). Mean time since diagnosis in years was 12.1 (10.6) and mean Expanded Disability Status Scale (EDSS) was 4.1 (1.1).
|
People with MS, n |
17 |
|
|
Age, mean (SD) |
41.6 (10.6) |
|
|
Sex, n (%) |
M |
6 (35) |
|
F |
11 (65) |
|
|
Years since diagnosis, mean (SD) |
12.1 (10.6) |
|
|
EDSS score, mean (SD) |
4.1 (1.1) |
|
|
Healthy controls, n |
12 |
|
|
Age, mean (SD) |
41.9 (8.3) |
|
|
Sex, n (%) |
M |
9 (75) |
|
F |
3 (25) |
Lower concentrations of glutamate are found at baseline in the thalami of people with highly active MS
A statistically significant difference in the concentration of glutamate was found between the two groups (7.67±0.3456 in HCs and 6.55±0.232 in pwMS, p=0.016). No significant difference was found between the two groups using other metabolites.

|
Metabolite |
Healthy controls (n=10) |
People with MS (n=15) |
95% CI |
|
Cho |
1. 69±0.0826,n=9 |
1.75±0.25, n=12 |
-0.232 – 0.216 |
|
Glu* |
7.67±0.346, n=6 |
6.55±0.232, n=8 * |
-2.00 – 0.253 |
|
Ins |
3.98±0.250, n=7 |
4.45±0.281, n=14 |
-0.452 – 1.380 |
|
tCr |
34±0.134, n=10 |
5.42±0.150, n=14 |
-0.350 – 0.510 |
|
tNAA |
8.60±0.134, n=9 |
8.46±0.178, n=14 |
-0.656 – 0.375 |
Baseline thalamic glutamate concentrations in pwMS correlate negatively with total lesion volumes
Baseline glutamate concentrations in pwMS negatively correlated with T1 scan total lesion volumes
(n=8; r=-0.80, p=0.017; Figure 6). No other thalamic metabolite correlated with lesion volumes. Lesion volumes in HCs (n=6) were assumed to be zero and are depicted in Figure 6, but this parameter was excluded from statistical analyses. No lesions were found in the thalami of pwMS in this study.
Glutamate concentration correlated even more strongly with left hemisphere lesion volumes (p=0.0091), an expected finding given that the left thalamus was used as the study’s ROI. The correlation was least significant when using right hemisphere lesion volumes (p=0.030). These results are reported in Table 3.

|
Sampled lesion load location |
r, correlation coefficient |
p-value |
|
Left hemisphere |
-0.84 |
0.0091 |
|
Right hemisphere |
-0.75 |
0.030 |
|
Both hemispheres/Total |
-0.80 |
0.016 |

Thalamic glutamate concentrations increase following natalizumab treatment
Glutamate concentrations measured in the thalami of pwMS increased significantly (p=??[LP7]) between the 10 and 56 weeks (n=12 pairs of data-points) follow-up scans. At 56 weeks, no significant difference between the pwMS and HC groups was recorded, suggesting that glutamate levels had normalised[LP8]. No significant difference in glutamate concentration was recorded between baseline and 10 weeks follow-up scans (n=7 pairs of data-points) and between baseline and 56 weeks follow-up (n=7 pairs of data-points).[LP9]

This observational study used proton magnetic resonance spectroscopy (1H-MRS) to compare metabolite concentrations in 17 pwMS and 12 HCs. Study findings indicate a lower baseline concentration of glutamate in the thalami of pwMS compared to HCs. In pwMS this correlated negatively with total baseline brain lesion volume, which supports our initial hypothesis that thalamic MRS specifically measuring glutamate can be used as a surrogate for global central nervous system pathology. An increase in glutamate concentrations was recorded following natalizumab treatment between 10 and 56 weeks of follow-up. To our group’s knowledge, this is the first 1H-MRS study to identify baseline cross-sectional differences in thalamic glutamate, correlate glutamate concentrations with total lesion volumes, and report longitudinal changes in thalamic glutamate following natalizumab treatment.
Thalamic glutamate is a potential surrogate for total brain neuronal damage in highly active MS
Glutamate, the chief central nervous system excitatory neurotransmitter is mainly synthesized from glutamine31,32. In addition to its neurotransmitter role, glutamate concentration is closely linked to the Kreb’s cycle, which reflects the cell’s metabolic activity. Previous proton MRS studies in MS reported higher levels of glutamate in lesioned white matter of pwMS compared to HCs33,34. One of these studies also reported lower levels of glutamate in lesioned grey matter regions34. The limitation of using white or grey matter lesions as ROIs is the high heterogeneity of these brain regions. With regards to WMLs, their definition includes- among others- active, inactive and remyelinating lesions. As for grey matter, this can be affected by exposure to cytokines from meningeal follicle-like structures or, similarly to WMLs, demyelination13,35,36. Current MRS imaging is unable to discriminate between these different pathologies. Therefore, metabolite concentrations obtained from these ROIs are likely to reflect the aforementioned local pathological changes, rather than global MS pathology. In contrast, the potential advantage of thalamic MRS is that the thalamus is rarely affected by local inflammation in MS37,38. Given that it is a subcortical hub highly connected with numerous other brain areas, this study hypothesised that the thalamus could be used as a biomarker of total brain neuronal damage in highly active MS. Two results in our study support this hypothesis: the decreased concentration of glutamate in pwMS and the negative correlation between glutamate and total brain lesion volume. Lesion volumes in MS have been found to correlate with axonal loss39 and disability40. Moreover, glutamate is mainly found in synaptic vesicles, therefore the decreased thalamic glutamate recorded in pwMS in this study could represent neuronal degeneration and synapse loss.
Thalamic glutamate increases following natalizumab treatment
Between 10 and 56 weeks of natalizumab treatment our group recorded a significant increase (p=??,) in the concentration of thalamic glutamate in pwMS. At the end of the follow-up period, glutamate levels normalised, with no significant difference being recorded between pwMS and HC groups. No significant differences in glutamate concentration were found between baseline and 10 (n=x pairs?) and baseline and 56 weeks (n=x pairs?)[LP10] follow-up scans. It can be hypothesised that the limited sample size of pairs of data-points between baseline and 56 weeks follow-up glutamate prevented us from recording an existing statistically significant difference. With regards to changes in glutamate between baseline and 10 weeks, there could be a significant change in glutamate concentration within this timeframe, which was not picked up due to our limited sample size. It also cannot be excluded that thalamic MRS may take longer to respond to treatment.
Previous published literature has shown lower glutamate concentrations in lesioned white matter of pwMS at baseline, which increased following treatment with natalizumab41. This effect can be attributed to the anti-inflammatory proprieties of natalizumab. By preventing production of nitrogen oxide and reactive oxygen species by macrophages, the drug could reduce axonal damage otherwise caused by these compounds42,43.
Study limitations
The algorithm used my SPM8 is incapable of accurately differentiating between the brighter grey and surrounding white matter, as the image intensity in the thalamus is very close to the intensity of white matter. Therefore the software records a higher white matter proportion in the thalamus than the true one. It should be however noted that this inaccuracy in measuring white/grey matter ratio should not cause any systematic error that would affect overall results.
The study’s HCs were adequately age-matched but poorly gender-matched to pwMS. Previous studies however reported no significant differences in any of the metabolite concentrations in the brain between different genders44. Therefore, no correction for a gender effect was made.
The HC group only had a baseline scan, with no longitudinal data recorded. A useful longitudinal control group may be untreated pwMS. The absence of such a control group is currently however a common limitation, as people with highly active MS are nearly always on treatment. Having no information on the natural history of thalamic MRS in pwMS, it is difficult to interpret the significance of longitudinal changes in glutamate seen in this study.
Lastly, albeit the thalamus is seldom affected by inflammatory activity in pwMS, the presence of inflammatory lesions has been previously described45. Such lesions are a confounding factor as they directly influence measured metabolite concentrations. However, based on T1, double inversion recovery pulse and phase sensitive inversion recovery sequences, no thalamic lesions were observed in our study.
Future work
Studies with larger sample sizes are needed to confirm our baseline findings, as well as to confidently interpret longitudinal changes in glutamate concentrations following natalizumab treatment. The presence of a pwMS untreated control group is not justifiable on ethical and legal grounds, however future research could use pwMS on less effective medication such as interferon-beta as a longitudinal control group. Histology studies investigating thalamic glutamate expression in post-mortem MS samples would enhance understanding of the cellular and molecular basis for the phenomena observed in this study.The correlation between thalamic glutamate and total lesion load is mainly associated with WMLs. Future research is warranted to test for correlations between thalamic MRS and other aspects of MS that contribute to disability. Detecting and quantifying GMLs will require high-field tesla MRI, as these are grossly underestimated on conventional MRI15,46.
Thalamic concentration of glutamate differs at baseline between pwMS and HCs. The correlation at baseline between glutamate in pwMS and total lesion volume suggests this metabolite could be used as a biomarker for total neuronal damage in the MS brain. This study’s results suggest that glutamate concentration increases significantly following natalizumab treatment and normalises at 56 weeks follow-up. Further research with bigger data samples is needed to investigate whether thalamic MRS can be used to predict long-term prognosis and long-term response to disease-modifying treatment.








